This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Hormonally up-regulated neu-associated kinase (HUNK) unveils a new function in cellular transport regulation
Endocytosis is a crucial cellular process that facilitates cells to take up cargos by enclosing them in membrane-bound vesicles, which are transported to different parts of the cell via the endosomal system and deliver cargos on the cytoskeleton and enable dynamic contact between various subtypes of endosomes or with other organelles, such as the lysosomes.
Endocytosis is classified into several mechanistic and morphological pathways, including clathrin-mediated endocytosis (CME) and caveolae-mediated endocytosis (CavME). However, the mechanisms and regulators involved in cargo uptake and delivery are still incompletely understood.
The CavME is a dynamin-dependent pathway via caveolae, which are invaginations of the plasma membrane enriched in cholesterol as well as sphingolipid and are generated by caveolins and CAVINs to facilitate the endocytosis of various substances, including toxins, growth factors, and bovine serum albumin (BSA). The specificity of endocytic pathways for particular cargo options and subsequent transporting routes remains poorly understood.
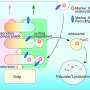
Siyuan Jiang et al. focused on the crucially endocytic process, which facilitates cells to take up cargo by enclosing them in membrane-bound vesicles. The expression profile analysis of HUNK-knockout cells is enriched in "endocytosis" and "lysosome" pathways, indicating that HUNK plays a key role in endocytosis.
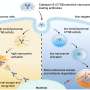
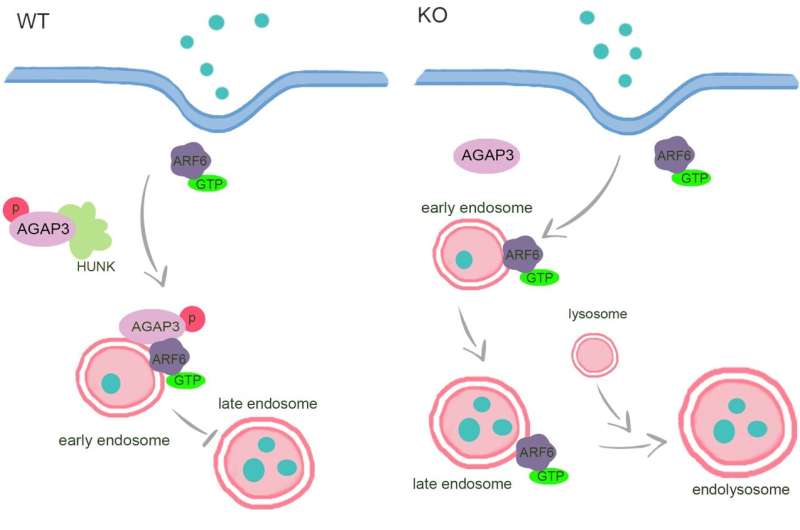
They utilized endocytic markers, inhibitors, and siRNAs to assess the function of HUNK on endocytosis signaling pathways. The results showed that HUNK selectively inhibits CavME and the cargo preferential delivery to lysosomes, which is dependent on its kinase activity.
Furthermore, they observed that lysosomes in knockout cells were larger in size and greater in number compared to control cells, demonstrating enhanced maturation of endolysosomes. Then they identified AGAP3 as a real binding partner of HUNK, and HUNK phosphorylates AGAP3 at the S396 site, which is critical for endolysosome formation and recruitment of AGAP3 to early endosomes, which negatively regulates the activity of ARF6.
This, in turn, leads to a reduction in the transition of early/late endosomes and the formation of endolysosomes.
Furthermore, HUNK-depleted colorectal cancer cells exhibited more efficient internalization of albumin-bound paclitaxel, enhancing tumor cytotoxicity. The combination of an inhibitor of HUNK and albumin-bound paclitaxel demonstrated improved therapeutic effects. This extends the understanding of HUNK in endocytosis and its potential implications in diseases such as cancer.
This team found that HUNK selectively inhibits the cargo uptake and traffic to lysosomes in the Caveolar pathway via phosphorylating AGAP3 to inactivate ARF6 in a kinase-dependent manner. Given the central role of endocytosis in the uptake of nanomedicines, this research provides new insights for improving the efficacy of nanoparticles in therapeutic applications.
The findings are published in the journal Science Bulletin.
More information: Siyuan Jiang et al, HUNK inhibits cargo uptake and lysosomal traffic in the caveolar pathway via the AGAP3/ARF6, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.11.053
Provided by Science China Press