This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Breaking an electrolyte's charge neutrality
Plant vascular circulation, ion channels, our own lymphatic network, and many energy harvesting systems rely on the transport of dissolved salt solutions through tortuous conduits. These solutions, or electrolytes, maintain a positive or negative charge that's vital to how the system functions. However, this charge balance depends on the properties of the channel that contains the fluid.
In a study published in The European Physical Journal E, Paolo Malgaretti, of the Helmholtz Institute Erlangen-Nürnberg for Renewable Energy/Forschungszentrum Jülich, Germany, and his colleagues, now derive equations that describe how local electrical charge in electrolytes changes in channels with varying cross sections, at equilibrium. The result could help to predict pathways for charged particles in biological and technological systems.
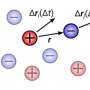
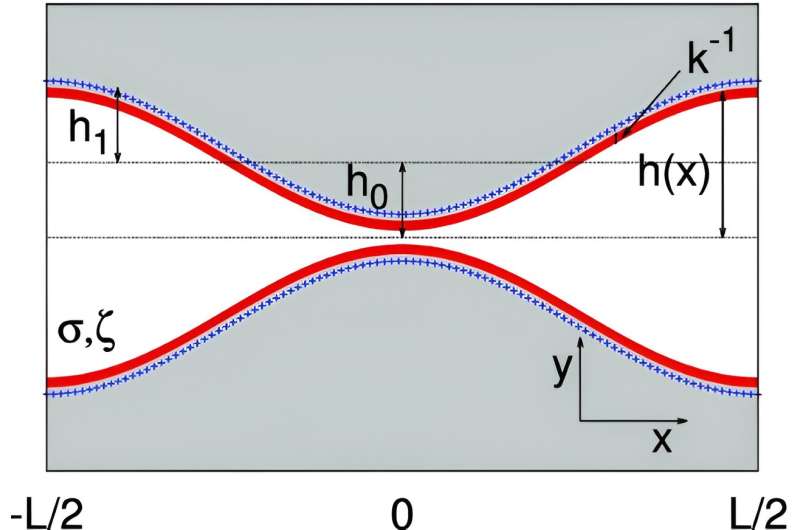
When an electrolyte solution is contained between two plates, theory says that the total electrical charge in the liquid should match that on the plates. However, Malgaretti and his team's observations show that when the plates approach a separation of fewer than 10 nanometers from one another, this charge balance breaks down. Moreover, novel dynamics appear for electrolytes moving through asymmetric pores or channels of varying diameters.
To capture the interplay between geometry and local electrolyte charge balance, Malgaretti and his team performed calculations of an electrolyte embedded between corrugated channel walls. They found that the local charge was broken whenever the cross-section of the channel changed. The researchers say that the onset of this excess charge results entirely from the interplay between channel geometry and electrostatic forces and is comparable to the total charge that built up on the walls.
The finding applied to both planar and cylindrical geometries, and to insulating and conducting channel walls. It could be used to predict corrections to the energetic pathways experienced by charged tracer particles, that are induced by the local excess charge.
More information: Paolo Malgaretti et al, Local electroneutrality breakdown for electrolytes within varying-section nanopores, The European Physical Journal E (2024). DOI: 10.1140/epje/s10189-024-00408-9
Provided by SciencePOD