This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers present 'domino effect' sensing mechanism to detect amine approaching picomolar level
Accurate and sensitive detection of amines is crucial in industrial safety, environmental monitoring and clinical diagnosis. Ongoing research and development of sensing materials offer promise for enhancing the sensitivity and selectivity of amine detection. Optical/fluorescent amine-detecting materials are particularly promising due to their easy manipulation, naked-eye readout, and portability.
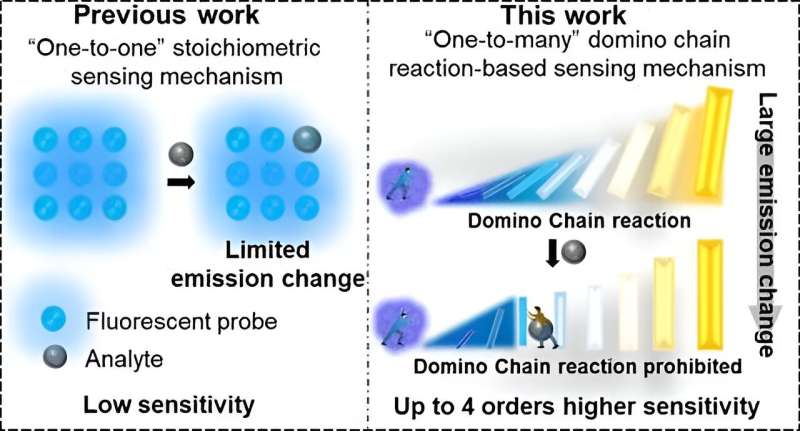
However, the reported fluorescent probes have suffered from moderate sensitivity due to inherent limitations in their "one-to-one" stoichiometric sensing mechanism. Typically, one amine molecule interacts with only one (or, at most, several) fluorescent molecules, causing a negligible impact on the overall emission of the system. Achieving a detectable signal change requires a significant number of amine molecules, giving rise to low sensitivities.
In a study published in the Journal of the American Chemical Society, the research group led by Prof. Huang Weiguo from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences developed a domino chain consisting of one fluorescent molecule (e.g., PTF1) and up to 40 non-emissive polymer chains (pPFPA) comprising more than 1,000 repeating units (PFPA).
The researchers found that the fluorophore PTF1 acts as the domino trigger, interacting with the surrounding polymer repeating units (PFPA, the following domino blocks) through polar-π interactions, leading to an amplified yellow fluorescent signal at 570 nm. Upon exposure to amines, the PTF1-pPFPA-based domino chain is rapidly dismantled, restoring the blue emissions at 455 nm assigned to intrinsic PTF1, thus creating an ultrasensitive method for amine detection.
The pentafluorophenyl motif is known for its electron-withdrawing properties. Conversely, amines are strong electron-donating compounds and strong bases. Upon mixing, the lone pair electrons on the "N" atom of Triethylamine (TEA) could readily communicate with the electron-deficient pendant groups on pPFPA, leading to an inductive effect that alters the electron cloud distribution among PFPA units and disrupts the original through-space conjugation (TSC) along the pPFPA chain.
Density functional theory (DFT) calculation revealed that the binding energy (ΔG) of pPFPA with TEA is -15.5 kcal mol-1, implying a strong affinity and interaction between TEA and pPFPA. The amines cut off the TSC along the domino if they embed among the PFPA repeating units or inhibit the charge transfer between PTF1 and pPFPA if they are in direct proximity to PTF1 fluorophores. In either condition, the established PTF1-pPFPA supramolecular domino chain will be severed and the original amplified yellow fluorescent signals will be substantially suppressed.
This study presents a "domino effect" sensing mechanism, which provides a general approach for chemical detection.
More information: Jiamao Chen et al, In Situ Optical Detection of Amines at a Parts-per-Quadrillion Level by Severing the Through-Space Conjugated Supramolecular Domino, Journal of the American Chemical Society (2024). DOI: 10.1021/jacs.3c11480
Journal information: Journal of the American Chemical Society
Provided by Chinese Academy of Sciences