This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Study reveals molecular mechanisms behind hibernation in mammals

Researchers have shed light on the molecular mechanisms underlying hibernation, publishing their findings today as a Reviewed Preprint in eLife.
Their research, in small and large hibernating mammals, is described by the editors as an important study advancing our knowledge of the role of myosin structure and energy consumption on the molecular mechanisms of hibernation, backed by solid methodology and evidence. The findings also suggest that myosin—a type of motor protein involved in muscle contraction—plays a role in non-shivering thermogenesis during hibernation, where heat is produced independent of the muscle activity of shivering.
Hibernation is a survival strategy used by many animals, characterized by a state of deep dormancy and profound reductions in metabolic activity, body temperature, heart rate and respiration. During hibernation, animals rely on stored energy reserves, particularly fats, to sustain their bodily functions. The metabolic slowdown allows hibernators to conserve energy and endure long periods of food scarcity and harsh environmental conditions during winter. However, the underlying cellular and molecular mechanisms behind hibernation remain incompletely understood.
Smaller hibernating mammals experience extended bouts of a hypo-metabolic state called torpor, which significantly decreases their body temperature and is punctuated by spontaneous periods of interbout euthermic arousals (IBA)—where they temporarily raise their body temperature to restore some physiological functions, such as eliminating waste and eating more food.
This contrasts with larger mammals, whose body temperature is much less reduced during hibernation and remains fairly consistent. Skeletal muscle, which comprises around half of a mammal's body mass, plays a key role in determining their heat production and energy use.
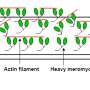
"Until recently, energy consumption in skeletal muscles was thought to be primarily linked to the activity of myosin, which is involved in muscle contraction. However, there is growing evidence that even when they are relaxed, skeletal muscles still use a small amount of energy," explains lead author Christopher Lewis, a postdoctoral researcher at the Department of Biomedical Sciences, University of Copenhagen, Denmark.
"Myosin heads in passive muscles can be in different resting states: the 'disordered-relaxed,' or DRX state, and the 'super-relaxed,' or SRX state. Myosin heads in the DRX state use up ATP—the energy currency of the cell—between five to ten times faster than those in the SRX state," Lewis adds.
Lewis and colleagues hypothesized that changes in the proportion of myosin in the DRX or SRX states may contribute to the reduced energy use seen during hibernation. To test this, they took skeletal muscle samples from two small hibernators—the thirteen-lined ground squirrel and the garden dormouse—and two large hibernators—the American black bear and brown bear.
First, they looked to establish whether the myosin states, and their respective ATP consumption rates, were different between active periods and hibernation. They looked at muscle fibers taken from the two bear species during their active summer phase (SA), and their winter hibernation period.
They found no differences in the proportion of myosin in the DRX or SRX state between the two phases. To measure the rate of ATP consumption by myosin, they used a specialized test called the Mant-ATP chase assay. This revealed that there were also no changes in the energy consumption rates of myosin. This may be to prevent the onset of significant muscular wastage in bears during hibernation.
The team also conducted the Mant-ATP chase assay on samples taken from the small mammals during SA, IBA and torpor. As in the larger hibernators, they did not observe any differences in the percentage of myosin heads in the SRX or DRX formation between the three phases. However, they did discover that the ATP turnover time of myosin molecules in both formations was lower in IBA and torpor compared to the SA phase, leading to an unexpected overall increase in ATP consumption.
As small mammals undergo a more significant drop in body temperature during hibernation than large mammals, the team tested whether this unexpected increase in ATP consumption also occurred at a lower temperature. They reran the Mant-ATP chase assay at 8° C, compared to the ambient lab temperature of 20° C used previously. Lowering the temperature decreased DRX and SRX-linked ATP turnover times in SA and IBA, leading to an increase in ATP consumption.
Metabolic organs, such as skeletal muscle, are well known to increase core body temperature in response to significant cold exposure, either by inducing shivering or through non-shivering thermogenesis. Cold exposure caused an increase in ATP consumption by myosin in samples obtained during SA and IBA, suggesting that myosin may contribute to non-shivering thermogenesis in small hibernators.
The team did not observe cold-induced changes in myosin energy consumption in samples obtained during torpor. They suggest that this is likely a protective mechanism to maintain the low core body temperature, and wider metabolic shutdown, seen during torpor.
Finally, the researchers wanted to understand the changes that occur at the protein level during the different hibernating phases. They assessed whether hibernation affects the structure of two myosin proteins from the thirteen-lined ground squirrel, Myh7 and Myh2. Although they did not observe any hibernation-related changes in the structure of Myh7, they discovered that Myh2 underwent significant phosphorylation—a process crucial for energy storage—during torpor, compared to SA and IBA.
They also analyzed the structure of the two proteins in the brown bear, finding no structural differences between SA and hibernation. They therefore conclude that Myh2 hyper-phosphorylation is specifically associated with torpor, rather than hibernation in general, and propose that this serves to increase myosin stability in small mammals. This may act as a potential molecular mechanism to mitigate myosin-associated increases in skeletal muscle expenditure in response to cold exposure during periods of torpor.
eLife's editors note that some areas of the study warrant further study. Namely, the muscle samples were taken exclusively from the legs of the animals studied. Given the core body and limbs have different temperatures, investigating muscle samples from other areas of the body would further validate the team's findings.
"Altogether, our findings suggest that ATP turnover adaptations in DRX and SRX myosin states occur in small mammals like the thirteen-lined ground squirrel during hibernation in cold environments. In contrast, larger mammals like the American black bear show no such changes, likely due to their stable body temperature during hibernation," concludes senior author Julien Ochala, Associate Professor at the Department of Biomedical Sciences, University of Copenhagen. "Our results also suggest that myosin may act as a contributor to skeletal muscle non-shivering thermogenesis during hibernation."
More information: Christopher T. A. Lewis et al, Remodelling of skeletal muscle myosin metabolic states in hibernating mammals, eLife (2024). DOI: 10.7554/eLife.94616.1
Provided by eLife