This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A novel micrometer-thick porous coating with unparalleled biomarker detection abilities
Aging populations and the tendency to lead a more sedentary lifestyle in many parts of the world are thought to dramatically increase the number of people living with multiple chronic conditions. Moreover, climate change, as well as shifting patterns in land use and travel, keep increasing the risk of infectious diseases that can emerge and spread locally and globally.
Being able to diagnose the presence and courses of all these diseases rapidly poses a growing challenge to health care systems—one that can only be met with the help of effective point-of-care (POC) diagnostic tests beyond the doctor's office and advanced medical facilities.
POC testing brought numerous benefits to people during the COVID-19 pandemic, but this approach needs to become applicable much more broadly and enable doctors and patients to probe deeper into pathological conditions. Current POC diagnostic technologies only measure a single disease biomarker or sometimes several biomarkers belonging to the same class of molecules, such as different RNAs, proteins, or antibodies.
However, measuring multiple biomarkers from different molecular classes could inform more comprehensively about the state a disease is in, its severity and progression over time, and even account for person-to-person differences in how it develops.
Electrochemical biosensors, which convert a chemical signal in the form of a biomarker present in a small sample of biofluid, such as blood, saliva, or urine, to an electrical signal that corresponds in strength to the detected amount of the biomarker, could provide the answer to many POC diagnostic problems.
In principle, multiple sensors for different biomarker molecules can be combined in multiplexed sensor arrays, and, importantly, the fight against "biofouling," the formerly inevitable ruin of electrode surfaces by unspecific biological molecules contained in samples, has become avoidable by the engineering of thin antifouling coatings pioneered at the Wyss Institute at Harvard University.
Now, the research team at the Wyss Institute, together with several collaborating institutes in Korea, has moved electrochemical diagnostic sensing a critical step further toward its broader application by developing a new nanocomposite porous antifouling coating that with a thickness of one micrometer—the diameter of a bacterium—which is about 100-fold thicker than previous coatings.
The coating's increased thickness and an engineered porous network within it allowed the incorporation of much higher numbers of biomarker-detecting probes into sensors, and thus, up to 17-fold higher sensitivities than previous best-in-class sensors while also providing superior antifouling capabilities.
In their proof-of-concept study, the researchers have built sensors that combined the ability to detect COVID-19-specific nucleic acid, antigen, and host antibody biomarker targets in clinical samples with high sensitivity and specificity. Their findings are published in Nature Communications.
"Our novel thick porous emulsion coating directly addresses critical hurdles that currently prevent the wide-spread use of electrochemical sensors as central components of comprehensive POC diagnostics for many conditions," said last-author and Wyss Founding Director Donald Ingber, M.D., Ph.D.
"However, going far beyond that, it could also open up new opportunities for developing safer and more functional implantable devices and other health care monitoring systems at multiple disease fronts. Overcoming biofouling and sensitivity problems are challenges that impact many of these efforts."
Ingber is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children's Hospital and the Hansjörg Wyss Professor of Bioinspired Engineering at Harvard John A. Paulson School of Engineering and Applied Sciences.
Thicker coating, better detection
In 2019, the Wyss Institute's electrochemical sensor project published its first landmark paper that reported the first antifouling coating with unprecedented biosensing capabilities.
In a series of critical follow-up studies, the team grew the potential of electrochemical sensing by further advancing the coatings' nanochemistry to make electrodes even more sensitive toward biomarkers, adding important multiplexing capabilities, and developing cost-reducing fabrication methods.
The most advanced biosensors the team engineered in the Wyss' eRapid platform had a feature set that is already enabling their translation into some clinical settings.
However, the coating method the team used exposed the entire sensor chip to the nanocomposite solution and only allowed a relatively thin coating of around 10 nanometers to form on the entire sensor surface, which limited the sensors' functionality in several ways.
For example, the coating's thin diameter restricted the maximum amount of probe that could be loaded into it, which becomes especially critical in larger multiplexed sensors that still need to work with small sample volumes and even more so in efforts to miniaturize multiplexed sensors for their use in portable POC diagnostic devices.
"In this new study, we came up with a completely new solution for this problem that resulted in a 100-times thicker coating. Our new approach harnesses an ink-jet printing method that allows us to apply this thick coating very locally to individual sensor elements," said former Wyss Senior Scientist Pawan Jolly, Ph.D., who was instrumental in evolving the eRapid platform.
"This opens up new possibilities: first, we can include much higher amounts of biomarker-detecting probes in the coating and, in the future, the sensors in complex arrays can be individually addressed by applying nanocomposite chemistries to them that are specifically geared towards specific biomarker modalities."
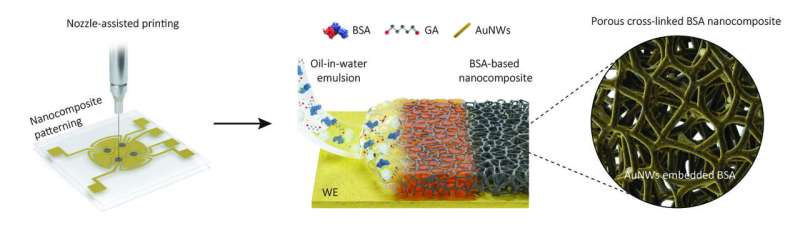
Instead of literally dipping electrochemical electrodes in a coating solution, as they did for their previous generation of sensors, researchers printed a layer of a dense oil-in-water emulsion through a fine nozzle onto electrodes. After evaporating the tiny oil bubbles, a 1 micrometer-thick coating remained on the electrode surface that consisted of cross-linked polymeric molecules of the blood protein albumin and contained interconnected pores and electron-conducing gold nanowires.
"The porous network in this nanocomposite coating dramatically increases the surface that can be used to attach specifically engineered biomarker-detecting probes to, and that at the same time is accessible to sample fluids. As a result, the detection sensitivity is significantly increased," explained first-author Jeong-Chan Lee, Ph.D., a Postdoctoral Fellow on Ingber's team.
"In addition, nozzle printing allows us to pattern the emulsion exclusively on the biomarker-detecting working electrode while keeping the neighboring reference electrode contained in each sensor free of it, which reduces non-specific electrical noise and enhances the specificity of our measurements."
Onward from COVID-19
The team re-purposed a previously developed combination of detection reagents for three COVID-19-related biomarkers to pattern a sensor electrode array using their newly developed coating technology: a CRISPR-enabled sensor for a SARS-CoV-2 RNA, a sensor specific for a SARS-CoV-2 capsid antigen, and a sensor for a virus-directed host antibody.
Tested with a collection of patient samples, the new sensor produced 3.75 to 17-fold enhanced detection sensitivities when compared to a previous one fabricated with the same detection systems and the team's best non-porous, much thinner coating. It also distinguished positive from negative samples with 100% accuracy (specificity).
"Electrochemical sensors with this next-generation coating would be ideal for monitoring viral outbreaks, vaccination responses, and understanding correlations among various biomarkers over the course of viral infections, and, in the future, they could be used for other diseases as well," said Lee.
More information: Jeong-Chan Lee et al, Micrometer-thick and porous nanocomposite coating for electrochemical sensors with exceptional antifouling and electroconducting properties, Nature Communications (2024). DOI: 10.1038/s41467-024-44822-1
Journal information: Nature Communications
Provided by Harvard University