This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Team develops light-powered catalyst to make hydrogen
A team from the UPC and the Catalan Institute of Nanoscience and Nanotechnology (ICN2) has designed an efficient and stable photocatalyst capable of producing hydrogen directly using sunlight. The results are published in the journal Nature Communications.
Hydrogen is essential for that energy transition, as long as it is produced from renewable sources (green hydrogen). It has long been known that electrons in some semiconductors can participate in chemical reactions when illuminated by sunlight.
This is the case with titanium dioxide, a cheap and harmless material that is widely used as a white pigment in paints, plastics, papers, inks and cosmetics. The excited electrons in titanium dioxide are capable of generating hydrogen from the protons in water and organic compounds. However, hydrogen production is very low because the electrons tend to relax rather than react, so the efficiency of the process is too low from a practical point of view.
This limitation can be overcome by bringing titanium dioxide into contact with metal nanoparticles, which act as electron filters, extending the life of the electrons in an excited state so that they can react and produce hydrogen. This allows us to achieve hundreds of times higher yields.
This study is a step forward for sustainable hydrogen production. It was led by Ramón y Cajal researcher Lluís Soler and professor Jordi Llorca from the ENCORE-NEMEN research group of the Department of Chemical Engineering and the Institute of Energy Technologies of the Universitat Politècnica de Catalunya—BarcelonaTech (UPC). They are also part of the Specific Center for Hydrogen Research (CER-H2).
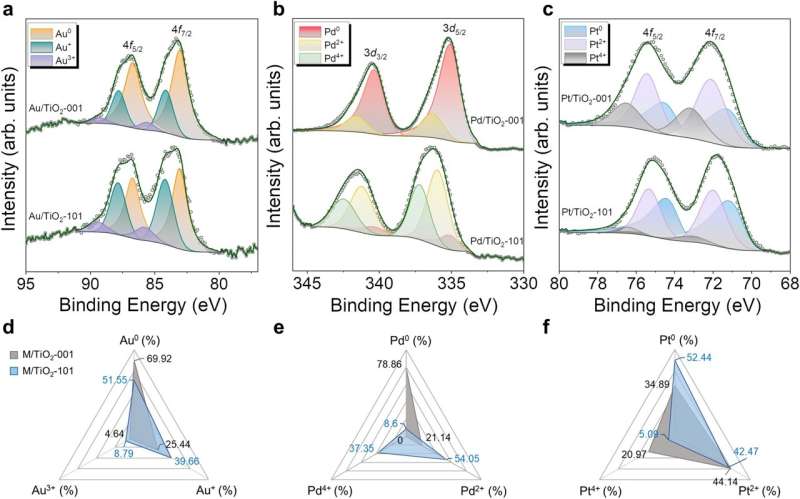
Using a mechanochemical process, the researchers deposited metal clusters on titanium dioxide nanoparticles of various morphologies and found that the different exposed crystallographic faces of titanium dioxide also play a key role in hydrogen production. Both the stability of photocatalysts and the strength of electron transfer between the semiconductor and the metal nanoparticles are strongly related to the semiconductor's exposed faces, which are responsible for atom mobility and aggregation.
The results are clear. When platinum clusters are deposited on octahedral titanium dioxide nanoparticles, a photocatalyst is obtained that produces higher quantities of hydrogen and, more importantly, is much more stable than any other combination. The study is a remarkable example of how nanotechnology can be applied to design new devices in the field of energy.
To understand the results, Ramón y Cajal researcher Claudio Cazorla from the UPC's Department of Physics has carried out quantum mechanical calculations to study the electronic structure of the photocatalysts, which were compared with the results of X-ray photoelectron spectroscopy obtained at the UPC's Research Center in Multiscale Science and Engineering. The center is located on the Diagonal-Besòs Campus, as is the Barcelona East School of Engineering (EEBE), where the researchers also teach.
The outcomes of this research will enable the design of new catalysts for the efficient and sustainable production of green hydrogen. Work is already underway at the UPC's at the Specific Center for Hydrogen Research to put these results into practice.
More information: Yufen Chen et al, Facet-engineered TiO2 drives photocatalytic activity and stability of supported noble metal clusters during H2 evolution, Nature Communications (2023). DOI: 10.1038/s41467-023-41976-2
Provided by Universitat Politècnica de Catalunya · BarcelonaTech (UPC)