This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Study demonstrates potency of synthetic antibiotic against serious chronic infections
A new synthetic antibiotic developed by University of Liverpool researchers is shown to be more effective than established drugs against "superbugs" such as MRSA, a new study shows.
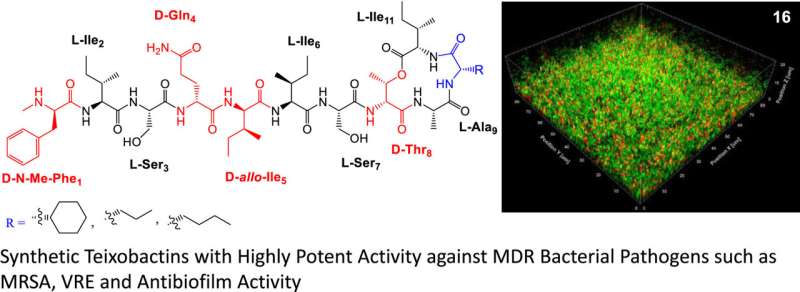
The study, "Development of teixobactin analogs containing hydrophobic, nonproteogenic amino acids that are highly potent against multidrug-resistant bacteria and biofilms," is published in the European Journal of Medicinal Chemistry.
The study demonstrates the potent activity of the antibiotic, teixobactin, against bacterial biofilms. Biofilms are clusters of bacteria that are attached to a surface and/or to each other—which are associated with serious chronic infections in humans.
Nearly 5 million people lose their lives due to antibiotic resistance-associated infections and millions more live with poor quality of life due to treatment failures. Antimicrobial resistance (AMR) is increasing and an AMR review commissioned by the UK Government has predicted that by 2050 an additional 10 million people will succumb to drug-resistant infections each year.
The development of new antibiotics which can be used as a last resort when other drugs are ineffective is a crucial area of study for health care researchers around the world.
This work builds on pioneering research by the University's Dr. Ishwar Singh, an expert in antimicrobial drug discovery and development and medicinal chemistry. A team of researchers led by Dr. Singh developed simplified synthetic versions of the natural molecule teixobactin, which is used by producer bacteria to kill other bacteria in soil.
They have tested a unique library of synthetic versions of the "game-changing" antibiotic, optimizing key features of the drug to enhance its efficacy and safety, plus enabling it to be inexpensively produced at scale.
For this latest study, the researchers designed and synthesized highly potent teixobactin analogs but swapped out key bottleneck building block L-allo-enduracididine with the commercially available low-cost simplified building blocks such as non-proteogenic amino acids. As a result, the analogs are now effective against a broad range of resistant bacterial pathogens including bacterial isolates from patients and bacterial biofilms.
This is another important step in adapting the natural teixobactin molecule to make it suitable for human use.
Dr. Ishwar Singh said, "Teixobactin molecules have the potential to provide new treatment options against multi-drug resistant bacterial and biofilm-related infections to improve and save lives globally. Our study provides a promising foundation for further research, and opens avenues to explore the application of teixobactin in various health-related biofilm contexts, including surgical site infections, implant-related surgeries and cystic fibrosis patients."
More information: Anish Parmar et al, Development of teixobactin analogues containing hydrophobic, non-proteogenic amino acids that are highly potent against multidrug-resistant bacteria and biofilms, European Journal of Medicinal Chemistry (2023). DOI: 10.1016/j.ejmech.2023.115853
Provided by University of Liverpool