This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Photochemistry and a new catalyst could make fertilizer more sustainable

Georgia Tech engineers are working to make fertilizer more sustainable—from production to productive reuse of the runoff after application—and a pair of new studies is offering promising avenues at both ends of the process.
In one paper, researchers have unraveled how nitrogen, water, carbon, and light can interact with a catalyst to produce ammonia at ambient temperature and pressure, a much less energy-intensive approach than current practice. The second paper describes a stable catalyst able to convert waste fertilizer back into nonpolluting nitrogen that could one day be used to make new fertilizer.
Significant work remains on both processes, but the senior author on the papers, Marta Hatzell, said they're a step toward a more sustainable cycle that still meets the needs of a growing worldwide population.
"We often think it would be nice not to have to use synthetic fertilizers for agriculture, but that's not realistic in the near term considering how much plant growth is dependent on synthetic fertilizers and how much food the world's population needs," said Hatzell, associate professor in the George W. Woodruff School of Mechanical Engineering. "The idea is that maybe one day you could manufacture, capture, and recycle fertilizer on site."
Producing ammonia at lower temperature, pressure
Nitrogen-rich ammonia is an essential fertilizer in global food production. Creating it requires significant petroleum-based energy, however, and it can only be done at 100 or so large-scale facilities worldwide.
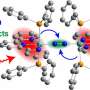
Hatzell and her Georgia Tech colleagues have uncovered the important role of molecules called carbon radicals for a low-energy approach that uses a light-reactive catalyst to fuse nitrogen and hydrogen into ammonia. They reported their findings in the Journal of the American Chemical Society Au (JACS Au).
Photochemical reactions are promising because they could use solar energy instead of fossil fuels and offer a more decentralized approach to ammonia manufacture. Typically, the necessary reaction requires temperatures around 400° Celsius and 100 times normal atmospheric pressure. Creating a process at ambient pressure and temperature—around 25° C—would be considerably easier.
The team, which included researchers from the School of Chemical and Biomolecular Engineering and the School of Civil and Environmental Engineering, used spectroscopy tools to show that light interacts with the photocatalyst to produce high-energy carbon molecules called carbon radicals.
"We found, surprisingly, that the nitrogen does not directly react at low temperatures. You really need the presence of carbon radical to aid the nitrogen fixation process," Hatzell said.
"It was really important for us to try to identify that reaction pathway, because without a clear understanding of how nitrogen and water results in the formation of ammonia, we really can't engineer systems and design new materials," she continued.
"By mapping this reaction pathway and understanding all the possible catalytic processes that can take place, we can now better engineer reactors and better design materials to accelerate the process."
The team used titanium dioxide as the photocatalyst in these experiments because it's well-studied and widely useful, but Hatzell said other materials might prove more effective at sparking the creation of ammonia in a photochemical reaction. This new understanding can help scientists begin to optimize the process.
Recycling fertilizer waste
The second study out of Hatzell's lab—published in ACS Energy Letters—is working at the opposite end of the fertilizer lifecycle. Significant amounts of nitrogen are wasted when fertilizer is applied to crops—perhaps as much as 80% goes unmetabolized by plants. This nitrate waste often ends up polluting groundwater.
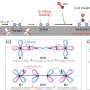
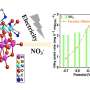
Hatzell worked with other Georgia Tech mechanical engineers and researchers at two national labs to create a palladium-copper alloy that reduces those nitrates back to nitrogen, which can be released harmlessly into the air or, one day, used to feed processes like the photochemical reaction in the JACS Au study to create new ammonia fertilizer.
"Not only is our catalyst good, but it's also stable for a very long period of time," Hatzell said. "Many researchers have come up with catalysts that get good conversion, but the catalysts aren't stable. We've created a highly-ordered alloy material that's effective, efficient, and also stable, which means that it would be able to work with these waste streams."
Both studies are the outgrowth of a concentration of expertise in the College of Engineering working to make advances in this area. They include contributions from researchers such as A.J. Medford, Seung Woo Lee, and Carsten Sievers.
They're also part of a broader effort, Hatzell and others at Tech help lead, that's working to reduce nitrogen pollution and instead create a circular nitrogen economy by capturing, recycling, and producing decarbonized nitrogen-based fertilizers.
"With that 10-year center, we're working to develop all of these individual processes and technologies," Hatzell said. "Then we'll figure out how to put them together and pilot them at wastewater treatment plants and agricultural sites."
More information: Po-Wei Huang et al, Formation of Carbon-Induced Nitrogen-Centered Radicals on Titanium Dioxide under Illumination, JACS Au (2023). DOI: 10.1021/jacsau.3c00556
Jeonghoon Lim et al, Atomically Ordered PdCu Electrocatalysts for Selective and Stable Electrochemical Nitrate Reduction, ACS Energy Letters (2023). DOI: 10.1021/acsenergylett.3c01672
Journal information: ACS Energy Letters
Provided by Georgia Institute of Technology