This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Deciphering molecular mysteries: New insights into metabolites that control aging and disease
In a significant advancement in the field of biochemistry, scientists at the Boyce Thompson Institute (BTI) and Cornell University have uncovered new insights into a family of metabolites, acylspermidines, that could change how we understand aging and fight diseases.
The study, recently published in Nature Chemical Biology, presents an unexpected connection between spermidine, a long-known compound present in all living cells, and sirtuins, an enzyme family that regulates many life-essential functions.
Sirtuins have been the subject of significant attention over the past two decades. Recent studies indicate that sirtuins play a crucial role in various age-related diseases. As a result, there is growing interest in the link between sirtuins and aging, making them a promising target for therapeutic interventions aimed at improving health span and longevity.
"We were excited to uncover this unexpected branch of cellular metabolism related to sirtuins," says senior author Frank Schroeder, a professor at BTI. "Discovering these previously uncharacterized spermidine derivatives provides insight into the inner workings of this critical pathway and brings us a step closer to understanding the physiological functions of mitochondrial sirtuins."
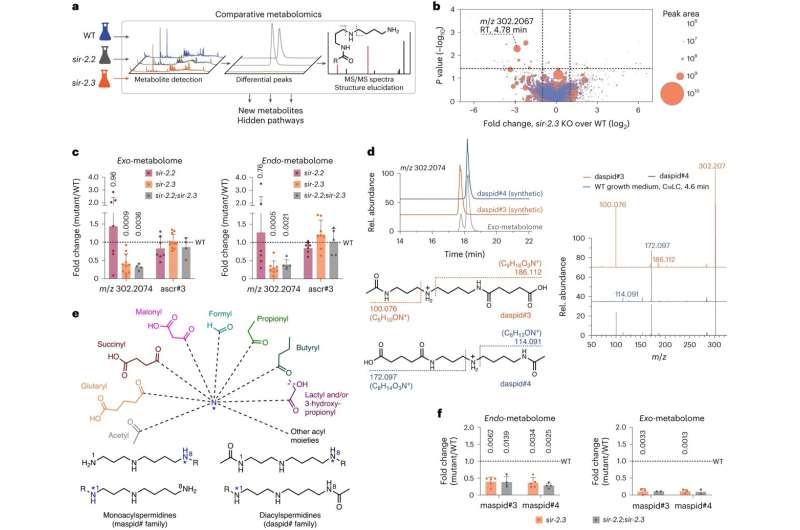
The researchers took an unbiased approach, comparative metabolomics, a methodology developed by the Schroeder lab for over a decade, to screen for sirtuin-dependent metabolic changes. The study revealed a novel family of metabolites called acylspermidines derived from modifications of diverse proteins, many of which play essential roles in growth and cell survival.
Following the discovery of sirtuin-linked acylspermidines in the simple organism C. elegans, the researchers further demonstrated that the same compounds are also present in mammals (including humans). Lastly, the research team demonstrates the direct impact of these metabolites on lifespan in C. elegans and cell proliferation in mammals.
"Important physiological functions are reflected in many molecular fingerprints, including tens of thousands of small molecule metabolites that remain to be discovered. This work is a step towards uncovering the biological roles and functions of the vast space of chemical dark matter in our bodies," says Bingsen Zhang, a graduate student in the Schroeder lab and first author of the study.
Future research will explore these findings' mechanisms and pharmacological aspects, particularly how acylspermidines affect lifespan, cell growth, and their potential interactions with other metabolic pathways.
"Nearly 350 years after spermidine was isolated and 100 years after its structure was understood, our work further advances the collective knowledge of the spermidine family, connecting it to other vital biochemical processes, including central energy metabolism and amino acid metabolism," adds Zhang.
More information: Bingsen Zhang et al, Acylspermidines are conserved mitochondrial sirtuin-dependent metabolites, Nature Chemical Biology (2024). DOI: 10.1038/s41589-023-01511-2
Journal information: Nature Chemical Biology
Provided by Boyce Thompson Institute