This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Researchers develop a low-cost catalyst for green hydrogen production
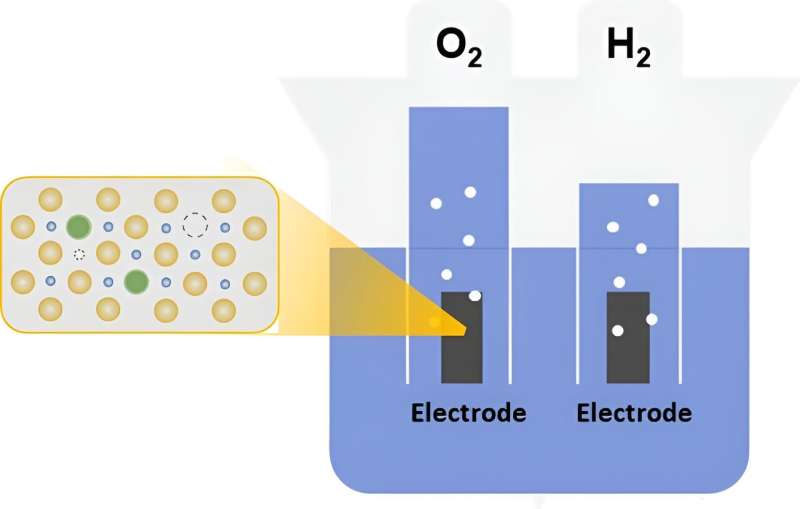
Electrolysis is a process that uses electricity to create hydrogen and oxygen molecules from water. The use of proton exchange membrane (PEM) and renewable energy for water electrolysis is widely regarded as a sustainable method for hydrogen production. However, a challenge in advancing PEM water electrolysis technology is the lack of efficient, low-cost, and stable catalysts for oxygen evolution reaction (OER) in acidic solutions during PEM water electrolysis.
While iridium-based catalysts are a potential solution, metallic iridium is rare and expensive in nature. Alternately, oxides of ruthenium (RuO2) offer a more affordable and reactive option, but they also suffer from stability issues. Therefore, researchers are exploring ways to improve the stability of the RuO2 structure to develop promising OER catalysts for the successful implementation of hydrogen production technology.
Now, in a recent study published in the Journal of Energy Chemistry, a group of researchers, led by Professor Haeseong Jang from the Department of Advanced Materials Engineering at Chung-Ang University, has developed a promising OER catalyst.
Denoted as SA Zn-RuO2, the catalyst comprises RuO2 stabilized by single atoms of zinc. Elaborating on their study, Prof. Jang says, "We were motivated by the need to find efficient and cost-effective alternative electrocatalysts for OER in PEM water electrolysis. Based on our study, we propose a dual-engineering strategy involving single atom Zn doping and the introduction of oxygen vacancies to balance high catalytic activity with stability during acidic OER."
The researchers synthesized SA Zn-RuO2 by heating an organic framework with ruthenium (Ru) and zinc atoms, forming a structure with oxygen vacancies (missing oxygen atoms that positively alter the properties) and Zn-O-Ru linkages.
These linkages stabilize the catalyst in two ways: by strengthening the Ru-O bonds and providing electrons from zinc atoms to protect ruthenium from overoxidation during the OER process. Furthermore, the improved electronic environment around the ruthenium atoms lowers the energies needed for molecules to stick to the surface, thus lowering the energy barrier for the reaction.
The resulting catalyst was more stable, with no apparent fall in reactivity, and significantly outperformed commercial RuO2. Moreover, it required less extra energy (low overpotential of 213 mV compared to 270 mV for commercial RuO2) and remained functional for a longer period (43 hours compared to 7.4 hours for commercial RuO2).
Due to its improved stability and features, the newly proposed SA Zn-RuO2 catalyst has the potential to influence the development of cost-effective, active, and acid-resistant electrocatalysts for OER. This, in turn, could help in reducing costs and enhancing the production of green hydrogen, aiding in a shift toward cleaner energy sources and advancements in sustainable technologies.
"We believe that this shift can revolutionize industries, transportation, and energy infrastructure and contribute to the efforts aimed at combating climate change and fostering a more resilient and environmentally conscious future. This is because accessible green hydrogen can have a transformative impact on societies by mitigating environmental impacts, creating jobs, and ensuring energy security through diversified and sustainable energy solutions," explains Prof. Jang.
In summary, the highly reactive and catalytically stable RuO2 catalyst for the acidic OER has increased durability and favorable characteristics and holds potential for guiding the design of robust and active non-iridium-based OER electrocatalysts for practical applications.
More information: Qing Qin et al, Tuning electronic structure of RuO2 by single atom Zn and oxygen vacancies to boost oxygen evolution reaction in acidic medium, Journal of Energy Chemistry (2023). DOI: 10.1016/j.jechem.2023.09.010
Provided by Chung Ang University