This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists innovate 'hook and slide' method to improve drug discovery
Historically, one of the primary ways organic chemists have explored and created compounds is by constructing a carbon skeleton and making modifications to its structure. But instead of building a carbon skeleton from scratch to make new compounds, UChicago scientists have developed a new method where they can insert atoms within an already existing carbon framework.
The innovation comes from a paper recently published in Science, by Rui Zhang, a fifth-year graduate student with the Guangbin Dong Lab. Zhang, with assistance from undergraduate Tingting Yu, developed a new "hook and slide" strategy that promises to optimize medicinal chemistry.
"It could lead to fast access to different drug candidates and thereforesave a lot of time in the drug discovery process," said Zhang.
Homologation
Back in May of 2021, Zhang began work on a problem having to do with how scientists create new molecules.
By tweaking the structure of molecules in a systematic way, scientists can explore how these changes affect the properties of the substances they are working with, providing a useful tool for tailoring molecules to specific needs in various applications. This is especially relevant in fields like drug development, where new lead-identification can potentially save lives.
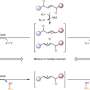
Specifically, Zhang wanted to successfully realize the homologation process with amides, a difficulty that had preoccupied the field and that had yet to be solved.
Homologation is one of the most important strategies for molecular modification.
In homologation, scientists build a family of related molecules where each member has a longer structure than the previous one. Once identified, they then add specific building blocks, often called methylene groups. As efficient as this process is, for years, when investigators tried to homologate amides, a compound found in proteins and formidable polymers like plastics, they were met with resistance and difficulty. Compared to other functional groups, amides have proven difficult because they are laconically inert, making them hard to activate, and thus manipulate.
Inspired by the technical challenge, Zhang was not satisfied with simply overcoming the difficulty, but finding new ways to do it well.
"There are no existing methods to homologate amides," said Professor Guangbin Dong, also an author on the study. "Our goal was to provide tunable homologation so we can insert a carbon unit of almost any length."
Hook and slide
Where previous methods failed to achieve the desired results, Zhang was able to complete the process and then some.
With what Dong describes as a "hook and slide strategy," they found the key to not only activate the bond, but to make the homologation process tunable.
Once Zhang nailed down the method for activation, he spent another two years honing the project, screening through different conditions, and finding more efficient ways to activate and create bonds.
With the publication in Science, he now feels that his work has finally paid off.
"We gained new knowledge about how to break this very inert carbon-carbon bond and we hope this can inspire the field to investigate more of this inert chemical bond activation," Zhang said. "We hope this tells the community that if you design the strategy well and it has a great catalyst, even an inert bond can be manipulated."
More information: Rui Zhang et al, Rhodium catalyzed tunable amide homologation through a hook-and-slide strategy, Science (2023). DOI: 10.1126/science.adk1001
Journal information: Science
Provided by University of Chicago