This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Scientists discover how to degrade and reform thermoset polymers without loss of function
A team of UK scientists has got a step closer to making several different types of plastic much easier to recycle using a method that could be applied to a whole range of difficult-to-recycle polymers, including rubbers, gels, and adhesives.
Thermoplastics and thermosets are two types of plastics that both consist of long chains of molecules called polymers but behave differently when heated.
Thermoplastics can be heated to high temperatures, poured into a mold then cooled to make the desired shape. They can subsequently be melted and reformed into other shapes when they are recycled. However, they can break when stretched or stressed.
In contrast, the polymer chains in thermoset plastics are crosslinked to form a network which makes them incredibly strong and flexible. They are often used in composite materials, paints, coatings, rubbers, and gels. Unfortunately, however, the crosslinks mean that the materials burn rather than melt when heated, making them much harder to break down and recycle.
Now, researchers at the University of Bath and the University of Surrey have developed a way of introducing degradable bonds into thermoset polymers to make them more easily recyclable.
Publishing in Polymer Chemistry, they made a series of polymer gels with breakable bonds incorporated into different parts of the structure and tested whether the properties changed after the gel was degraded and reformed.
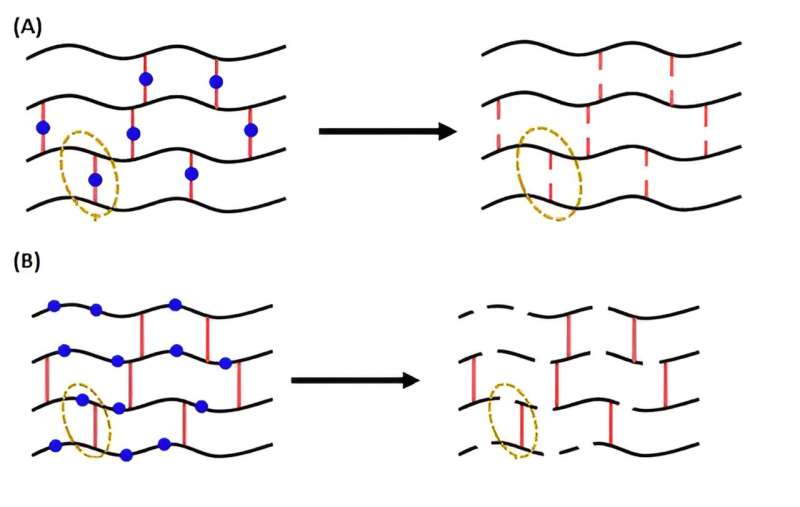
They found that while all the gels could be degraded to some extent, gels with breakable bonds in the polymer chains (B in the attached diagram) retained their properties much better when reformed, compared with the polymers that were broken down via the cross-linked bonds (A).
The researchers hope this model system can be applied to other types of polymers, including adhesives, sealants, and elastomers.
Dr. Maciek Kopeć, from the University of Bath's Department of Chemistry, said, "Thermosets are used widely in the commercial sector, in materials like resins and adhesives.
"Being able to make bonds reversible in these materials will increase their applications as well as make them more recyclable."
The researchers aim to create a general road map of the best locations for these breakable bonds, to understand better why some bonds break more easily than others, and to plan to optimize the system using other commercially used polymers.
The researchers are also looking at other applications of the work, including using crosslinked polymers as vehicles for controlled drug delivery systems.
More information: Frances Dawson et al, Strands vs. crosslinks: topology-dependent degradation and regelation of polyacrylate networks synthesised by RAFT polymerisation, Polymer Chemistry (2023). DOI: 10.1039/D3PY01008B
Provided by University of Bath