This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
New research reveals critical steps in Lassa virus ribonucleoparticle assembly and recruitment
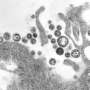
Lassa virus (LASV) is the pathogen that causes Lassa hemorrhagic fever, a disease endemic to West Africa, which causes approximately 5,000 deaths each year. At the CSSB Centre for Structural Systems Biology, the Uetrecht (CSSB, LIV, Uni Siegen), Kosinski (CSSB, EMBL) and Rosenthal (BNITM, CSSB) groups worked together to reveal the crucial role played by RNA in critical steps of the Lassa virus life cycle.
Their findings are published in the Journal of the American Chemical Society.
In the human body, 20,000 genes produce over one million different forms of proteins. The Lassa virus in comparison is miniscule as it is composed of only four proteins, known as L, NP, Z and GPC.
"We are trying to understand how these four proteins can cause such serious damage to human cells," explains the paper's first author Lennart Sänger. "The activities and expression of these proteins must be tightly regulated and the proteins must communicate efficiently with one another to take on different functions."
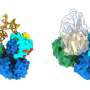
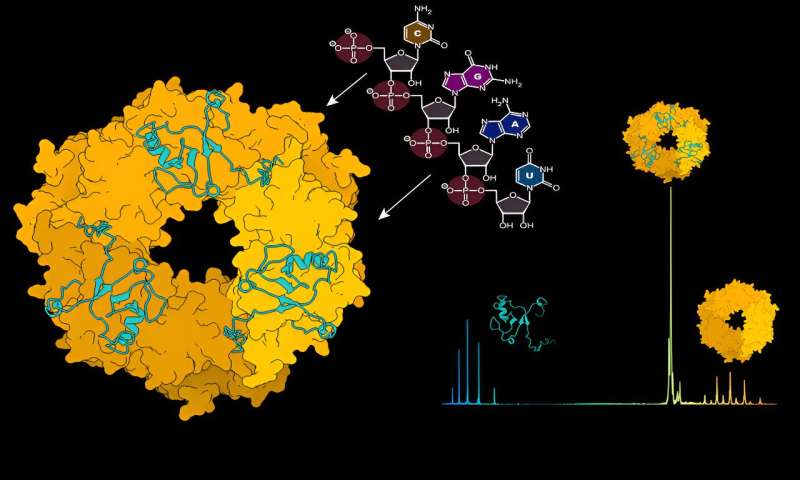
To protect and hide the virus from detection by the immune system, the nucleoprotein (NP) encloses the viral genome in a capsid. This capsid together with viral RNA and the L protein forms ribonucleoprotein complexes (RNPs).
To propagate infection, RNPs must continuously restructure themselves in order to enable viral genome replication and transcription. The researchers investigated the interactions between NP and viral RNA as well as the Z protein to gain a better understanding of the mechanism and dynamics of RNP formation and packaging into new viral particles.
Using structural mass spectrometry, a method that acts like a molecular scale by revealing the atomic weight of molecular interactions, the researchers examined the dynamics between NP and viral RNA. "Initially, the NP protein doesn't exist in a composition that can bind viral RNA," explains Charlotte Uetrecht, a CSSB group leader and expert in mass spectrometry techniques.
"A change needs to occur to enable this binding and we discovered that viral RNA can initiate this change by itself." The researchers identified RNA as the driver for the disassembly of ring-like NP trimers into monomers which are then able to form higher order RNA-bound NP assemblies.
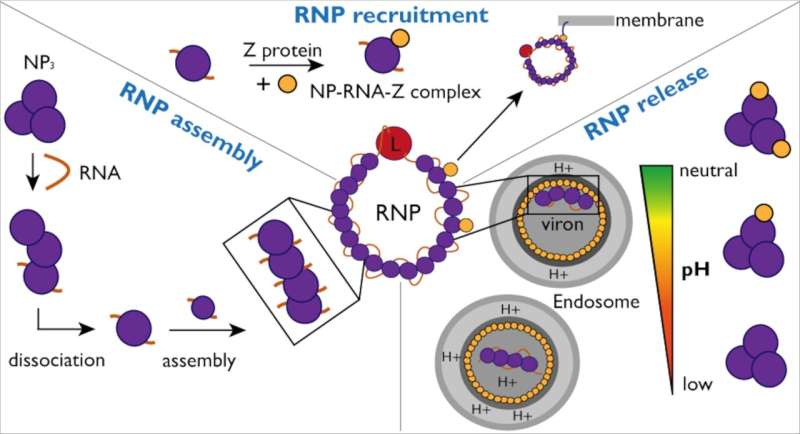
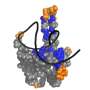
The researchers also investigated NP interaction with the Z protein in more detail. To facilitate this, the Kosinski group used AlphaFold to predict the NP-Z complex's interaction site. These predictions were then verified by researchers in the laboratory.
"Using artificial intelligence enabled us to quickly identify possible interactions and also enabled us to create mutants to verify our hypothesis," notes Jan Kosinski. The researchers were ultimately able to demonstrate that while NP binds Z independently of the presence of RNA, this interaction is pH-dependent.
"Overall, these findings help improve our understanding of RNP assembly, recruitment, and release in Lassa virus," explains Maria Rosenthal, a Lassa virus expert at the Bernhard Nocht Institute for Tropical Medicine and a CSSB associate member. In West Africa, 186 million people are predicted to be at risk of Lassa virus infection by 2030, and the World Health Organization recognizes Lassa virus as a dangerous and yet understudied pathogen.
"Understanding how Lassa virus functions may ultimately enable us to develop molecules which could inhibit the replication of this virus and treat Lassa fever," notes Rosenthal.
More information: Lennart Sänger et al, RNA to Rule Them All: Critical Steps in Lassa Virus Ribonucleoparticle Assembly and Recruitment, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c07325
Journal information: Journal of the American Chemical Society
Provided by CSSB Centre for Structural Systems Biology