This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers reveal evolutionary secret underlying the rise of seed plants
In a study published in Nature Plants, Chao Daiyin's group at the Center for Excellence in Molecular Plant Sciences of the Chinese Academy of Sciences and Lyu Shiyou's group at Hubei University have revealed, for the first time, the mystery behind the rise of seed plants from the perspective of specialized cell wall evolution.
Seed plants are the most advanced plant group in the world, accounting for two-thirds of all plant species and shaping the predominant flora of our world. However, the Earth was very different more than 300 million years ago during the Carboniferous period, when ferns were the dominant flora, with towering tree ferns dominating the ecological landscape. Most of the coal resources on Earth today came from fern plants of that period, hence the name Carboniferous.
However, paleontological research reveals a turning point at the end of the Carboniferous period, with Earth's climate suddenly becoming cold and arid. As a result, ferns began to decline, paving the way for the rise of seed plants. Nevertheless, this significant evolutionary event is marked by many unsolved mysteries, with one of the most important mysteries being: What specific advantages did seed plants evolve that allowed them to transition from a weaker position to a thriving one by the end of the Carboniferous period?
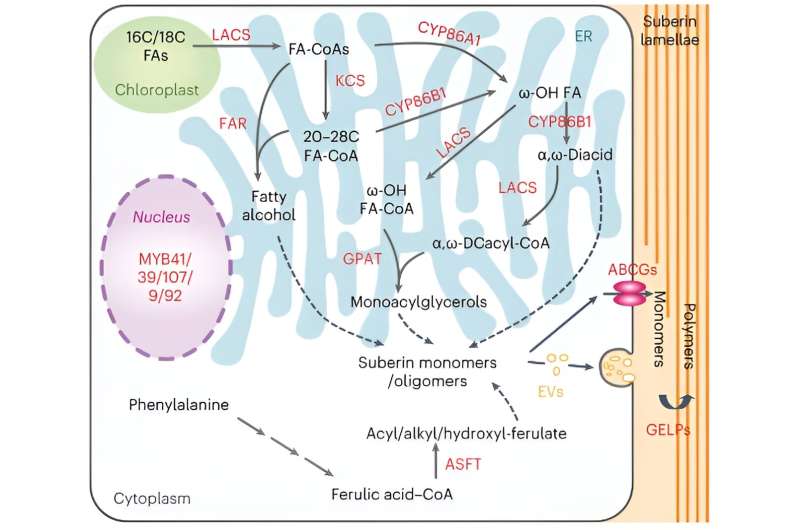
Roots are essential organs for absorbing and transporting water and mineral nutrients in plants, and the endodermis is the core of the root, controlling water and mineral transport. The endodermis cell wall features a hydrophobic, lignin-based Casparian strip tightly anchored to the endodermal cell membrane, thus forming a barrier to prevent the free diffusion of substances. Additionally, the suberin lamellae are specialized cell wall structures that envelop the entire surface of endodermal cells.
Research indicates that both the Casparian strip and suberin lamellae play essential roles in plant nutrient balance and water transport, but their functions are significantly different. Chao's group had previously made breakthroughs in understanding the formation and anchoring of the Casparian strip. However, the evolutionary basis of suberin lamellae and their role in plant evolution had not yet been resolved.
This study used a series of advanced cell biology and analytical chemistry techniques to conduct in-depth research on representative plant species from 18 different evolutionary nodes with the aim of unveiling the secrets of the origin of the Casparian strip and suberin lamellae.
Surprisingly, the researchers found that the Casparian strip exists in all vascular plants, including ferns, lycophytes, gymnosperms, and angiosperms, while suberin lamellae are only present in gymnosperms and angiosperms (both collectively referred to as seed plants).
This evidence suggests that the Casparian strip and suberin lamellae did not originate simultaneously; the former emerged from the common ancestor of all vascular plants, while the latter evolved in the common ancestor of seed plants. This finding challenges the longstanding assumption regarding suberin lamellae and offers new perspectives for studying the evolution of these structures.
To investigate how suberin lamellae evolved in the common ancestor of seed plants, the researchers conducted molecular evolutionary analyses of the genes involved in suberin lamellae formation and their homologs, with these results: Although most of these genes had evolved before the appearance of vascular plants, significant expansion occurred in the common ancestor of seed plants.
This expansion suggested that gene duplication likely led to functional innovations, thus enabling the genes responsible for synthesizing suberin lamellae to emerge in the common ancestor of seed plants.
To confirm this hypothesis, the researchers investigated homologous genes of the core MYB transcription factors involved in suberin formation in fern plants, lycophytes, gymnosperms, and angiosperms. These genes were found to be widespread in all these plant groups. However, a significant expansion of homologous genes occurred in the common ancestor of gymnosperms and angiosperms.
The researchers revealed that the expanded homologous genes in gymnosperms and angiosperms could initiate suberin lamellae formation, while the homologous genes in fern plants and lycophyte plants had no such function. This finding confirmed that the function of MYB transcription factors initiating suberin synthesis was acquired in seed plants through gene expansion.
As Earth's climate became dry in the late Carboniferous period, ferns began to decline and seed plants increased. Since suberin has waterproof properties, the researchers hypothesized that the emergence of suberin lamellae might have contributed to the drought adaptability of seed plants, thus promoting their rise after the onset of arid conditions. They later confirmed this hypothesis by using two Arabidopsis genetic materials with suberin defects, thereby demonstrating that suberin-deficient Arabidopsis was more sensitive to drought.
Furthermore, Raman spectroscopy and nuclear magnetic resonance revealed the crucial significance of suberin lamellae in enhancing the efficiency of vascular water transport. Specifically, since water molecules are capable of free diffusion across cell membranes in the absence of suberin lamellae, plants without suberin lamellae, such as ferns and horsetails, experience significant water leakage from endodermal cell membranes when subjected to osmotic stress, resulting in low transport efficiency.
Seed plants, with suberin lamellae fully enveloping their endodermal cells, almost completely block the free diffusion of water molecules. Thus, their water leakage rate under osmotic stress is only 1%–2% compared to fern plants and lycophyte plants. This waterproofing effect greatly enhances the efficiency of water transport in the vascular tissues of seed plants under drought conditions, thereby increasing their drought resistance.
Based on this, the researchers proposed a model for the rise of seed plants: In the moist climate of the Carboniferous period, fern plants with no suberin lamellae had higher water and nutrient absorption efficiency and were better adapted to the environment, thus causing them to thrive.
However, during the late Carboniferous period, the onset of a dry climate provided an advantage for seed plants that had evolved suberin lamellae. They possessed a more efficient water transport system and stronger drought tolerance, allowing them to gradual replace ferns and become the dominant life forms on Earth's surface.
This study not only unveils the mystery of the origin of the Casparian strip and suberin lamellae but also provides evidence, for the first time, that the emergence of suberin lamellae drove the rise of seed plants, based on a new perspective. Furthermore, it identifies the important role of suberin lamellae in plant adaptation to adverse conditions such as drought. As a result, this study has significant implications for enhancing plant drought resistance, elucidating plant salt and drought tolerance mechanisms, and developing drought-resistant crop varieties.
More information: Yu Su et al, The evolutionary innovation of root suberin lamellae contributed to the rise of seed plants, Nature Plants (2023). DOI: 10.1038/s41477-023-01555-1
Journal information: Nature Plants
Provided by Chinese Academy of Sciences