This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Engineering bacteria to biosynthesize intricate protein complexes
Protein cages found within microbes help its contents weather the harsh intracellular environment—an observation that has many bioengineering applications. Tokyo Tech researchers have recently developed an innovative bioengineering approach that uses genetically modified bacteria to incorporate protein cages around protein crystals. This in-cell biosynthesis method efficiently produces highly customized protein complexes, which could find applications as advanced solid catalysts and functionalized nanomaterials.
In nature, proteins can assemble to form organized complexes with myriad shapes and purposes. Thanks to the remarkable progress in bioengineering over the past few decades, scientists can now produce customized protein assemblies for specialized applications. For example, protein cages can confine enzymes that act as catalysts for a targeted chemical reaction. Similarly, protein crystals—structures composed of repeating units of proteins—can serve as scaffolds for synthesizing solid materials with exposed functional terminals.
However, incorporating (or "encapsulating") foreign proteins on the surface of a protein crystal is challenging. Thus, synthesizing protein crystals that encapsulate foreign protein assemblies has been elusive. So far, no efficient methods exist to achieve this goal, and the types of protein crystals produced are limited. But what if bacterial cellular machinery was the answer?
In a recent study, a research team from Tokyo Institute of Technology, including Professor Takafumi Ueno, reported a new in-cell method for encapsulating protein cages with diverse functions on protein crystals. Their paper, published in Nano Letters, represents a substantial breakthrough in protein crystal engineering.
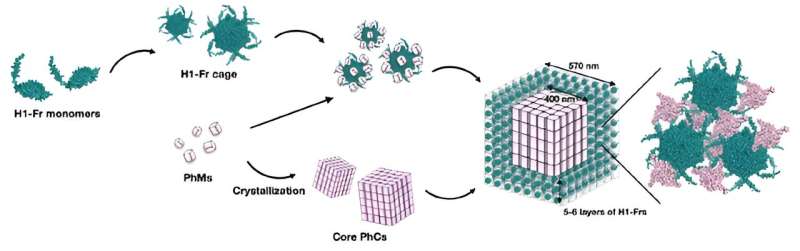
The team's strategy involves genetically modifying Escherichia coli bacteria to produce two main building blocks: polyhedrin monomer (PhM) and modified ferritin (Fr). On the one hand, PhMs naturally combine within cells to form a well-studied protein crystal called polyhedra crystal (PhC). On the other hand, 24 Fr units are known to combine to form a stable protein cage.
"Ferritin has been widely used as a template for constructing bio-nano materials by modifying its internal and external surfaces. Thus, if the formation of a Fr cage and its subsequent immobilization onto PhC can be performed simultaneously in a single cell, the applications of in-cell protein crystals as bio-hybrid materials will be expanded," explains Prof. Ueno.
To immobilize the Fr cages into PhC, the researchers modified the gene coding for Fr to include an α-helix(H1) tag of PhM, thus creating H1-Fr. The reasoning behind this approach is that the H1-helixes naturally present in PhM molecules interact significantly with the tags on H1-Fr, acting as "recruiting agents" that bind the foreign proteins onto the crystal.
Using advanced microscopy, analytical, and chemical techniques, the research team verified the validity of their proposed approach. Through various experiments, they found that the resulting crystals had a core–shell structure, namely a cubic PhC core about 400 nanometers wide covered in five or six layers of H1-Fr cages.
This strategy for the biosynthesis of functional protein crystals holds much promise for applications in medicine, catalysis, and biomaterials engineering. "H1-Fr cages have the potential to immobilize external molecules inside them for molecular delivery," says Prof. Ueno.
"Our results indicate that the H1-Fr/PhC core–shell structures, displaying H1-Fr cages on the outer surface of the PhC core, can be individually controlled at the nanoscale level. By accumulating different functional molecules in the PhC core and H1-Fr cage, hierarchical nanoscale-controlled crystals can be constructed for advanced biotechnological applications."
Future works in this field will help us realize the true potential of bioengineering protein crystals and assemblies. With any luck, these efforts will pave the way to a healthier and more sustainable future.
More information: Thuc Toan Pham et al, Displaying a Protein Cage on a Protein Crystal by In-Cell Crystal Engineering, Nano Letters (2023). DOI: 10.1021/acs.nanolett.3c02117
Provided by Tokyo Institute of Technology