This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
AI chemist synthesizes catalyst for oxygen production from Martian meteorites: One step closer to Mars immigration?
Immigration to and living on Mars have long been depicted in science fiction. But before that dream turns into reality, there is a hurdle humans have to overcome—the lack of chemicals such as oxygen essential for long-term survival on the planet. However, the recent discovery of water activity on Mars is promising.
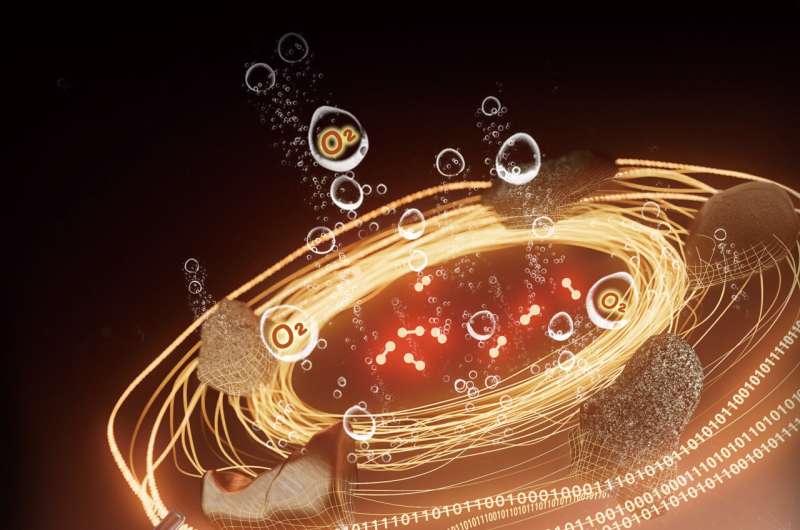
Scientists are now exploring the possibility of decomposing water to produce oxygen through electrochemical water oxidation driven by solar power with the help of oxygen evolution reaction (OER) catalysts. The challenge is to find a way to synthesize these catalysts in situ using materials on Mars, instead of transporting them from the Earth, which is costly.
To tackle this problem, a team led by Prof. Luo Yi, Prof. Jiang Jun, and Prof. Shang Weiwei from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS), recently made it possible to synthesize and optimize OER catalysts automatically from Martian meteorites with their robotic artificial intelligence (AI)-chemist.
Their research was published in Nature Synthesis.
"The AI chemist innovatively synthesize[d] OER catalyst using Martian material based on interdisciplinary cooperation," said Prof. Luo Yi, leading scientist of the team.
In each experimental cycle, the AI chemist first analyzes the elemental composition of the Martian ores using laser-induced breakdown spectroscopy (LIBS) as its eyes.
Then, it carries out a series of pretreatments on the ores, including weighing in the solid-dispensing workstation, preparing feedstock solutions in the liquid-dispensing workstation, performing separation from the liquid in the centrifugation workstation, and achieving solidification in the dryer workstation.
The resulting metal hydroxides are treated with Nafion adhesive to prepare the working electrode for OER testing at the electrochemical workstation. The testing data are sent to the computational 'brain' of the AI chemist in real-time for machine learning (ML) processing.
The AI chemist's 'brain' employs quantum chemistry and molecular dynamics simulations for 30,000 high-entropy hydroxides with different elemental ratios and calculates their OER catalytic activities via density functional theory. The simulation data are used to train a neural network model for rapidly predicting the catalysts' activities with different elemental compositions.
Finally, through Bayesian optimization, the 'brain' predicts the combination of available Martian ores needed for synthesizing the optimal OER catalyst.
So far, the AI chemist has created an excellent catalyst using five types of Martian meteorites under unmanned conditions. This catalyst can operate steadily for over 550,000 seconds at a current density of 10 mA cm-2 and an overpotential of 445.1 mV. A further test at -37 °C, the temperature on Mars, confirmed that the catalyst can steadily produce oxygen without any apparent degradation.
Within two months, the AI chemist has completed the complex optimization of catalysts that would take 2,000 years for a human chemist.
The team is working to turn the AI chemist into a general experiment platform for various chemical synthesis without human intervention. The paper's reviewer remarked, "This type of research is of wide interest and is under rapid development in organic/inorganic material synthesis and discovery."
"In the future, humans can establish an oxygen factory on Mars with the assistance of an AI chemist," said Jiang. Only 15 hours of solar irradiation is needed to produce sufficient oxygen concentration required for human survival. "This breakthrough technology brings us one step closer to achieving our dream of living on Mars," he said.
More information: Jun Jiang, Automated synthesis of oxygen-producing catalysts from Martian meteorites by a robotic AI chemist, Nature Synthesis (2023). DOI: 10.1038/s44160-023-00424-1. www.nature.com/articles/s44160-023-00424-1
Journal information: Nature Synthesis
Provided by Chinese Academy of Sciences