This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Study provides a new strategy for building high-performance small-molecule NIR-II PTAs
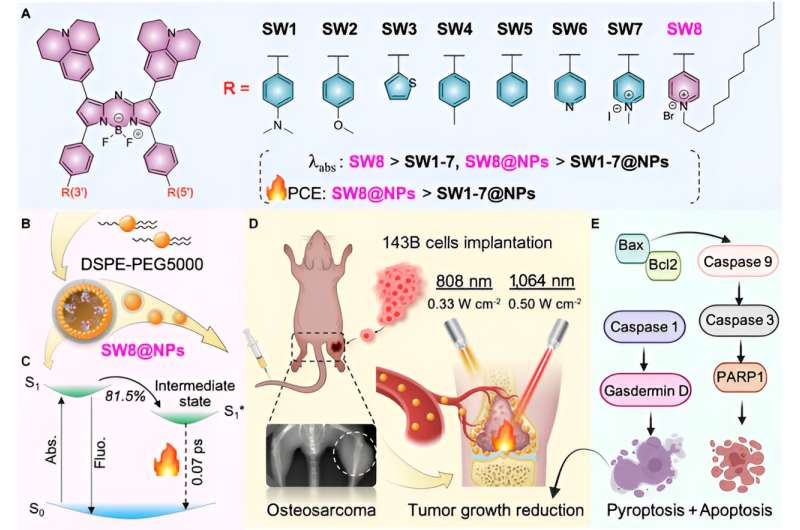
Recently, the team of Academician Huang Wei, Professor Li Lin and Professor Hu Wen Bo from the School of Northwestern Polytechnical University developed an ultra-efficient NIR-II photothermal agent for 1,064 nm laser-mediated photothermal treatment of osteosarcoma.
The study, "Acceptor Engineering Produces Ultrafast Nonradiative Decay in NIR-II Aza-BODIPY Nanoparticles for Efficient use. Osteosarcoma Photothermal Therapy via Concurrent Apoptosis and Pyroptosis," was published in Research.
Cancer treatment is still one of the biggest challenges facing people today, despite substantially better medical technology. In recent years, the development of near-infrared (NIR) photothermal agents (PATs), which are molecular targeted drugs for photothermal therapy (PTT), has emerged as a new research hotspot.
Compared with other bands of light, NIR light has better biological penetration ability, and can be used for mild PTT of deep tissue when combining with appropriate PATs. The majority of mice model experiments are still in the subcutaneous tumor therapy stage, which is limited by the shallow penetration depth of NIR-I light and cannot remove deep tumor tissue in the body.
However, the NIR-II light's penetration depth increases and the deep tumor PTT is expected to achieve clinical application. At present, there are many studies on PTAs , and various new materials are frequently developed. The factors that affect the function of PTAs include absorption wavelength, size and surface modification.
Although different types of PTAs show unique advantages, a photothermal material that integrates many advantages such as high photothermal conversion efficiencies (PCEs), a long absorption wavelength, strong biosafety and good water solubility needs to be explored.
Small-molecule PTAs with intense NIR-II absorption and high PCEs are promising candidates for treating deep-seated tumors such as osteosarcoma. To date, the development of small-molecule NIR-II PTAs has largely relied on fabricating donor–acceptor–donor (D–A–D/D') structures and limited success has been achieved.
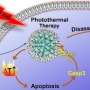
Herein, through acceptor engineering, a donor–acceptor–acceptor (D–A–A')-structured NIR-II aza-boron-dipyrromethene (aza-BODIPY) PTA (SW8) was readily developed for the 1,064 nm laser-mediated phototheranostic treatment of osteosarcoma.
Changing the donor groups to acceptor groups produced remarkable red-shifts of absorption maximums from NIR-I regions (~808 nm) to NIR-II ones (~1,064 nm) for aza-BODIPYs (SW1 to SW8). Furthermore, SW8 self-assembled into nanoparticles (SW8@NPs) with intense NIR-II absorption and an ultrahigh PCE (75%, 1,064 nm).
This ultrahigh PCE primarily originated from an additional nonradiative decay pathway, which showed a 100-fold enhanced decay rate compared to that shown by conventional pathways such as internal conversion and vibrational relaxation. Eventually, SW8@NPs performed highly efficient 1,064 nm laser-mediated NIR-II PTT of osteosarcoma via concurrent apoptosis and pyroptosis.
This work not only illustrates a remote approach for treating deep-seated tumors with high spatiotemporal control but also provides a new strategy for building high-performance small-molecule NIR-II PTAs.
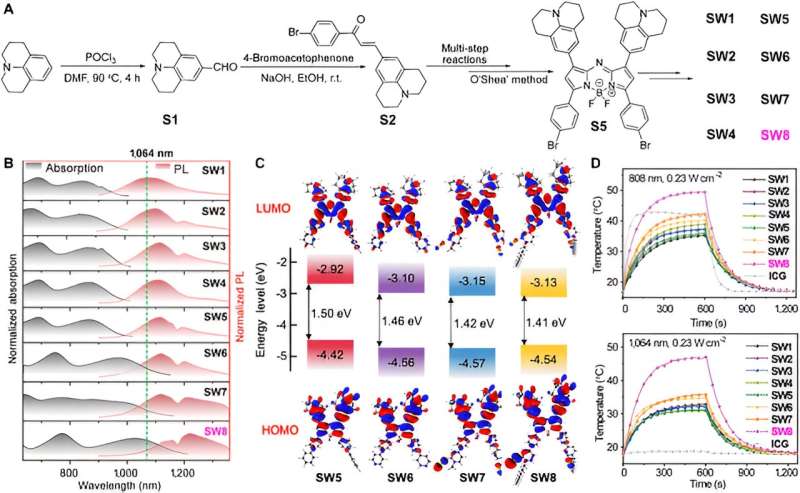
First, the researchers synthesized a series of organic small-molecule PTAs based aza-BODIPY. Notably, remarkable red-shifts for the absorption and PL spectra occurred from SW5 to SW6, where the electron-donating benzene donor (D') was changed to the pyridine acceptor (A').
Further increase in the electron deficiency of A' caused more bathochromic wavelengths from SW6 to SW8, especially the red shift of absorption wavelength is obvious, whereas the electron-donating moiety (from SW1 to SW5) exhibited no pronounced wavelength shift. In addition, the introduction of alkyl chains enhanced the J-aggregation of molecules, which is manifested by a red shift of emission wavelength from SW6 to SW8. Time-dependent density functional theory (TD-DFT) calculations revealed a gradual decrease in the HOMO-LUMO energy gap from SW5 to SW8, consistent with the red-shifted spectra.
Notably, the LUMO energy levels for SW5–8 reduced in order, whereas the HOMO energy levels were almost unchanged. This phenomenon indicates that the acceptor segments at the 3- and 5-positions of aza-BODIPY significantly reduced the energy gaps, offering an alternative approach to construct new organic small-molecule NIR-II materials.
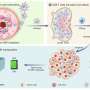
Then this article selects the most advantageous absorption/emission wavelength SW8 for self-assembly. SW8 was encapsulated into an amphiphilic matrix to form water-soluble nanoparticles (SW8@NPs). The PCE of SW8@NPs under 1,064 nm laser irradiation was determined to be as high as 75%, which is a remarkable improvement compared to those of SW1-7@NPs.
Furthermore, ultrafast spectroscopic studies ascribed this ultrahigh PCE to a nonradiative intermediate state. This dark intermediate depleted up to 80% of the excited population with a high decay rate of 1.3 × 1013 s-1 over conventional nonradiative decay channels such as internal conversion, resulting in the ultrahigh PCE.
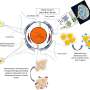
Finally, in order to study the biocompatibility of SW8@NPs, osteosarcoma cell 143B was treated with SW8@NPs. The results showed that SW8@NPs could be efficiently cellular uptake by the 143B cells with low dark toxicity and high phototoxicity. The apoptosis level was significantly increased after 808 nm and 1,064 photothermal images were taken. X-ray imaging were used to monitor orthotopic tumor growth. the tumors were irradiated with laser for 10 mins and repeated every other day for 12 days.
The curve of the volume tendency for the "SW8@NPs +1,064 nm laser" group demonstrated complete tumor eradication during the 12 days of monitoring. By contrast, the other five treatments failed to suppress tumor growth, with an average increase in tumor volume of 4- to 5-fold nm laser irradiation. Compared with 808 nm laser, 1,064 nn laser can penetrate 15 mm thick muscle tissue, effectively stimulate SW8@NPs heat production. Further studies orthotopic 143B tumor-bearing mice showed that SW8@NPs had high accumulation and strong photothermal effect at the tumor site. Continuous irradiation of tumor regions for 10 mins using an 808 (0.33 W cm-2) or 1,064 nm laser (0.5 W cm-2) was performed 24 h after SW8@NPs injection.
Moreover, histology and immunohistochemical assays showed that the tumor structure in the "SW8@NPs+1,064 nm" group was severely damaged, and the parenchymal cells disappeared in large numbers and appeared vacuolar, and the TUNEL results showed that the level of apoptosis was significantly increased. Western blotting was used to analyze apoptosis-associated proteins (Bax, Bcl2, Caspase 9, Caspase 3, and PARP1).
In the "SW8@NPs+1,064 nm" group, the overall phosphorylation level of apoptotic protein was increased, and apoptosis was activated irradiation. Together, the results revealed, for the first time, that the SW8@NPs-mediated NIR-II PTT exerted antitumor effects mainly by stimulating concurrent apoptosis and pyroptosis.
In this study, they reported on the design of a novel organic small-molecule PTA (SW8) and self-accessibility nanoparticles (SW8@NPs) with a high PCE (75%) in the NIR-II window (1,064 nm). Molecular excited-state dynamics analyzing showed that this ultrahigh PCE primarily originated from an additional nonradiative decay pathway.
A series of in vitro and in vivo experiments demonstrated for the first time that superior NIR-II PTT could effectively induce concurrent apoptosis and pyroptosis in osteosarcoma tissues. The researchers suggest that the design of organic small-molecule PTAs based on rational principles in the NIR-II window will benefit the practical clinical applications of photothermal activations and treatments in the future.
More information: Zhenxiong Shi et al, Acceptor Engineering Produces Ultrafast Nonradiative Decay in NIR-II Aza-BODIPY Nanoparticles for Efficient Osteosarcoma Photothermal Therapy via Concurrent Apoptosis and Pyroptosis, Research (2023). DOI: 10.34133/research.0169
Journal information: Research
Provided by Research