This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Liquid reagent can rapidly inactivate coronavirus for faster, safer and potentially life-saving testing
A Queensland research collaboration has identified a simple, quick way to safely kill coronavirus in patient diagnostic samples and speed up processing for PCR tests—by using a unique sample preparation liquid.
The study published in Frontiers in Microbiology involved the University of the Sunshine Coast (UniSC), QIMR Berghofer Medical Research Institute, The University of Queensland and BioCifer, a Queensland based medical biotech company.
UniSC Associate Professor of Molecular Engineering Joanne Macdonald said the liquid reagent—developed by UniSC researchers—had the potential to save lives by speeding up diagnostic testing, leading to faster test results and faster treatment.
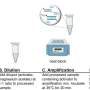
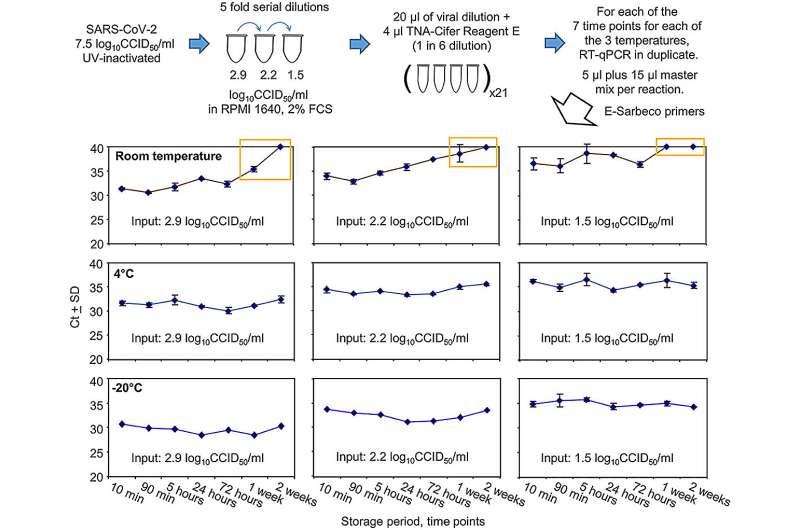
"The study showed that the unique composition of the reagent could extract RNA in patient samples in little as five to 10 minutes—and importantly, rapidly inactivate SARS-CoV-2 to make testing safer," she said.
"It's a simple three-step protocol. No magnetic beads, no spin columns, no elution. Just mix, hold for a short time at room temperature, and your sample is ready for PCR," said Dr. Macdonald, who is also BioCifer's co-founder and current Chief Technology Officer.
"While many people think that SARS-CoV-2 is 'old news,' it's important to realize that it is still the third leading cause of death in Australia after heart disease and dementia, and early detection continues to be critical, particularly in our elderly and vulnerable populations."
The reagent, known as TNA-Cifer Reagent E, was originally developed at UniSC's Center for Bioinnovation. QIMR Berghofer performed the inactivation testing experiments at its SARS-CoV-2 PC3 Biosecurity facility. The University of Queensland assisted by testing its ability to detect SARS-CoV-2 in patient samples.
Professor Andreas Suhrbier, who leads QIMR Berghofer's Inflammation Biology Group, said faster, safer and cheaper, yet accurate, diagnosis clearly had many advantages.
"Not least of which is prompt initiation of treatment, such as nirmatrelvir/ritonavir, for high risk individuals," Professor Suhrbier said.
"It is very gratifying to see our state-of-the-art biosecurity facility, that we operate thanks to generous philanthropic donations from The Brazil Family Foundation and others, help Queensland biotech produce new products that will transform diagnosis of hazardous pathogens," he said.
QIMR Berghofer research officer and BioCifer consultant Dr. Daniel Rawle said the findings paved the way for diagnosis to be performed in resource poor settings.
"This is a wonderfully simple way to both quickly kill SARS-CoV-2 in test samples and speed up processing that can also be used outside formal pathology laboratory settings using portable PCR machines," Dr. Rawle said.
A potential new tool for managing disease outbreaks
The TNA-Cifer reagent has also been demonstrated, in other studies, to potentially enable safe, rapid onsite testing of other diseases such as dengue, Nipah and Hendra viruses.
Dr. Macdonald said the reagent has potential to help manage outbreaks of disease by preventing testing backlogs and providing real-time information.
"For SARS-CoV-2, we show that by combining rapid sample processing and testing with Australian-made portable PCR testing equipment from Bio Molecular Systems, we now have a full platform for rapid decentralized testing," she said.
"This could be critical when needing to scale up rapid testing," she said, "By using existing PCR tests and our reagent, we could quickly and safely test samples on-site rather than having to ship them back to the laboratory where backlogs can occur."
DMTC Head of Government Relations Harry Baxter said that through its Health Security Systems Australia division, DMTC and its partners were advancing Australia's manufacturing, research and development capabilities in areas relevant to defense, national security and health security.
"In this case, the acceleration of the accurate detection of bacteria and viruses outside of the laboratory environment provides a crucial capability advantage for deployed Australian military personnel, and benefits public health service delivery," Baxter said.
More information: Nina M. Pollak et al, Rapid inactivation and sample preparation for SARS-CoV-2 PCR-based diagnostics using TNA-Cifer Reagent E, Frontiers in Microbiology (2023). DOI: 10.3389/fmicb.2023.1238542
Journal information: Frontiers in Microbiology
Provided by University of the Sunshine Coast