This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Drug-delivery technique with vessel-targeted gold nanoparticles shows growing promise for brain cancer treatment
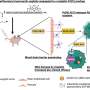
A technique developed by University of Texas at Dallas and UT Southwestern Medical Center researchers to deliver medication through the blood-brain barrier has shown promise in a preclinical study for treating glioblastoma, the most common human brain cancer.
The researchers demonstrated the method in mice in a study published in Nature Communications.
Glioblastoma is an aggressive brain cancer that affects about 12,000 people annually in the U.S.; patients have a median survival of 15 to 18 months after diagnosis. Current treatments, which include surgery, chemotherapy and radiation, are largely ineffective. It is difficult to get chemotherapy into glioblastoma tumors because most medications will not pass through the blood-brain barrier, which is a unique property of blood vessels in the brain that restrict and actively prevent substances in the bloodstream from reaching the brain parenchyma.
The barrier acts like a highly selective filter and protective barrier for the brain, said co-corresponding author of the study Dr. Zhenpeng Qin, associate professor of mechanical engineering and Fellow, Eugene McDermott Professor in the Erik Jonsson School of Engineering and Computer Science.
"The biggest challenge to treat any brain disease is this barrier. It's amazing; it's only a micron thick, but it prevents 98% of molecules from getting inside the brain," Qin said. By comparison, the diameter of human hair is 70 microns.
Qin collaborated with UT Southwestern colleagues Dr. Robert Bachoo, co-corresponding author and associate professor of neurology and internal medicine, and Dr. Elizabeth Maher, professor of internal medicine and neurology. The research involved genetically engineered mice that had mutations found in human glioblastoma patients.
Qin's drug-delivery method relies on co-delivering medication with vessel-targeted gold nanoparticles, which are injected into the bloodstream. From an external source, researchers apply short laser pulses, which pass through the mouse skull and activate the gold nanoparticles. This activation generates thermomechanical waves and briefly makes the blood-brain barrier permeable, allowing medication to reach its target. In their experiments, researchers used paclitaxel, a chemotherapy drug used to treat ovarian, breast and lung cancers, which was abandoned for potential use against brain cancer because, on its own, the drug molecule does not cross the barrier.
The study demonstrated that the new approach overcame the barrier, although years of research will be needed before the method can be tested in humans. Further preclinical studies are ongoing.
"The tumors shrank in size, and we expanded survival by more than 50%," Qin said. "We hope this will lead to expanded therapeutic options for treating diseases in the brain and central nervous system."
More information: Qi Cai et al, Optical blood-brain-tumor barrier modulation expands therapeutic options for glioblastoma treatment, Nature Communications (2023). DOI: 10.1038/s41467-023-40579-1
Journal information: Nature Communications
Provided by University of Texas at Dallas