This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Using nickel foam to enhance the performance of lithium-sulfur batteries
Lithium-sulfur (Li-S) batteries are considered important devices for powering movable equipment, but there are still some challenges that limit their application, such as how to obtain a cathode for high sulfides absorption and rapidly conversion.
To spur more research into the high-performance Li-S battery, researchers in China provided a new Ni@HsGDY /MoS2/Ni3S2 composite material to alleviate the shuttle effect, volume expansion, and other issues in lithium-sulfur batteries.
They published their work on Sep. 26 in Energy Material Advances.
"The booming progress of electric vehicles demands next-generation energy storage technologies with high energy density, low cost, and longevity," said Fushen Lu, a professor at the college of chemistry and chemical engineering in Shantou university.
"Lithium-sulfur batteries are identified as a promising energy storage system because of their high ultrahigh energy density and large theoretical capacity. However, they are limited by the poor electronic conductivity of sulfur, volume changes of the cathode, and shuttle effect."
Lu explained that the conversion of polysulfides (Li2Sn, 4 ≤ n ≤ 8) is a complicated multiphase transformation during the charge-discharge period. The dissolvable lithium polysulfides (LiPSs) diffuse through the porous separator to the negative electrode and react with Li metal to form non-dissolvable Li, causing "shuttle effect" and leading to a deteriorative discharge capacity and cycling performance. This is one of the main defects seriously hindering the large-scale commercial applications of Li-S batteries.
"Currently, the obstacles of Li-S batteries are mainly overcome through the design of electrodes and electrolytes," Lu said. "To enlarge the absorption capacity of lithium polysulfides and enhance the generation of active sulfur during the charge-discharge period, the electrodes are always composed of the porous interlayer with high catalytic activity."
Carbon materials with nonpolar surface hardly catalyze the conversion of soluble lithium polysulfides. According to Lu, some metal oxide nanoparticles or organic-inorganic hybrids were employed to anchor lithium polysulfides for boosting the absorption and conversion of lithium polysulfides. However, these materials perform sluggish redox kinetics of the high-sulfur-loading Li-S battery due to the accumulation of lithium polysulfides in the interlayers. The active species were utilized at low level.
To obtain an efficient cathode, the combination of carbon materials and inorganic functional material seems a viable strategy, said Lu. However, the catalytic compositions were usually covered by the intermediate layer, which decreased the conversion of polysulfides, resulting in the final failure to achieve the synergistic effect of the two functions.
"In past years, lots of effort has been devoted to the cathodes of lithium-sulfur batteries with excellent chemical adsorption of polysulfides and high catalytic efficiency. It is still an urgent issue to find a feasible strategy to integrate multiple respective functions to accelerate the conversion of the polysulfides." said Lu.
"The graphdiyne (GDY) was known as a new carbon allotrope with planar structure and unique properties. The butadiyne linkages (-C≡C-C≡C-) to benzene rings lead to lower atom density and give rise to natural pores, which could play a key role in the lithium-sulfur batteries."
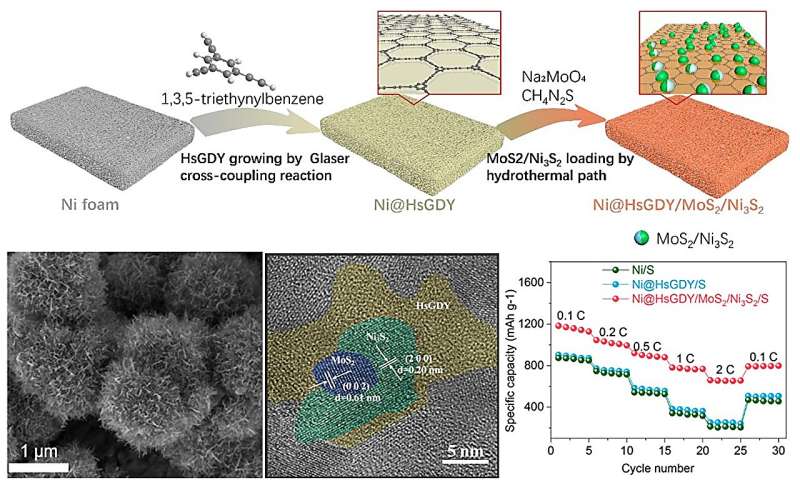
"In our work, a new strategy is proposed to enhance the performance of lithium-sulfur batteries by growing 3D HsGDY (hydrogen substituted graphdiyne) layers on Ni foam via Glaser cross-coupling reaction to anchor MoS2/Ni3S2, enhancing the conductivity of sulfur-hosting material," said Lu.
"The 3D HsGDY framework enables the fast adsorption of lithium polysulfides and the Ni3S2/MoS2 performs as the reaction center with a low charge transfer resistance."
Lu concluded that the Ni@HsGDY /MoS2/Ni3S2/S electrodes exhibit high performance in lithium-sulfur batteries, with large specific capacity and long-term stability at high current densities. The incorporation of HsGDY into cathode can promote the absorption and conversion of lithium polysulfide in the electrolyte, providing new ideas for obtaining high-energy density lithium-sulfur batteries.
"Lithium-ion batteries have been commercialized for decades. Their energy density have marginally increased in the past a few years, though considerable amount of research efforts have been spent," said Lu. "The research of HsGDY-containing Li-S battery is still in its infancy and significant investigations are still required to realize the practical applications."
More information: Jiao Xiang et al, HsGDY on Ni Foam for Loading MoS 2 /Ni 3 S 2 to Enhance the Performance on Lithium–Sulfur Batteries, Energy Material Advances (2023). DOI: 10.34133/energymatadv.0054
Provided by Beijing Institute of Technology Press Co.