This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Chemical engineers draft a roadmap for research into metallic 'sponges' for clean hydrogen
Metal organic frameworks (MOFs) could deliver a major efficiency boost to the photocatalytic production of clean hydrogen. Chemical engineers have drafted a comprehensive overview of the state of their field and a plan for where it needs to focus.
Clean hydrogen production remains an energy-intensive and therefore costly proposition, inhibiting the battle against global warming. Metal organic frameworks—in effect tiny molecular 'sponges'—look set to radically improve the efficiency of photocatalytic production of hydrogen due to their unique structural properties, but the research into the subject faces considerable challenges. A group of chemical engineers have produced an overview of the state of the field with a roadmap of where investigations should be focused to most likely achieve progress.
Their review paper was published in the journal Polyoxometalates on August 4, 2023.
Hydrogen will be necessary for the clean transition away from fossil fuels, whether as an energy storage mechanism, an input for clean fuels or as a clean fuel directly, or for decarbonized steel and ammonia production. But the hydrogen itself must be cleanly produced, from the splitting of water into its component parts.
Unfortunately, such water splitting is an energy hog, which drives up the cost of clean hydrogen production. If clean hydrogen is going to be competitive with dirty hydrogen production—typically via the splitting of methane, a greenhouse gas—then water splitting needs to achieve some significant increases to its efficiency.
One widely discussed efficiency-boosting option comes from photocatalytic water splitting with the assistance of metal organic frameworks, or MOFs.
First, the energy from sunlight activates the photocatalyst—a material that jumpstarts and speeds up the water splitting reaction. Next, imagine a Lego-like structure, but where the Lego bricks are instead made of metal clusters—a large group of metallic atoms—and the connectors (or "linkers") between them are organic molecules.
These structures form porous 3D networks that act sort of the way sponges do to absorb liquids into their pores. But these metal-organic 'sponges,' or more properly, metal organic frameworks (MOFs) are so small that they operate at the molecular level, allowing scientists to trap, store, or separate various gases and chemicals inside.
MOFs can be game-changers for photocatalytic water splitting due to their unique properties, particularly with respect to absorbing the sunlight that kicks off the whole photocatalytic water splitting process.
Research into the role of MOFs for photocatalytic water splitting has exploded in recent years, and so the authors felt it was time to produce a scientific review paper on the topic. Scientific review papers are like "best-of" music albums for science, gathering all the hit discoveries and insights on a topic into one comprehensive overview. They act as compasses for the scientific community, summarizing past research to guide future explorations and helping researchers build upon existing knowledge rather than reinventing the wheel.
The review paper first sets out the key advantages of MOFs here. Some MOFs can absorb sunlight and can then transfer the energy to other materials or use it directly to drive the water splitting reaction.
Moreover, the efficiency of a photocatalyst largely depends on its ability to excite electrons to jump a 'band gap' up from the valence level of an atom to its conduction level—where these excited electrons can now flow freely in an electric current. MOFs can be designed and modified to optimize their band gaps, making them more suitable for absorbing visible light.
"MOFs also have a large surface area due to their porous nature," said Huan Pang, one of the review paper's authors and a chemical engineer in the School of Chemistry and Chemical Engineering, Yangzhou University. "Think of all that internal surface area encapsulating the pores."
This extra surface area means that MOFs provide a greater number of locations where the water-splitting chemical reactions can take place—locations known as "active sites." More places for those reactions means greater efficiency in water splitting.
MOFs can also serve as supports for other photocatalytic materials, ensuring they remain stable and dispersed. This can prevent agglomeration (clumping together) of photocatalytic particles, which can reduce their efficiency.
"And one of the biggest advantages of MOFs is their sheer versatility," added Yang An, a co-author of the paper at the Institute for Innovative Materials and Energy at Yangzhou University. "Chemical engineers can customize the MOF structures by selecting different metals and organic linkers, allowing for the design of MOFs specifically tailored for efficient photocatalytic water splitting."
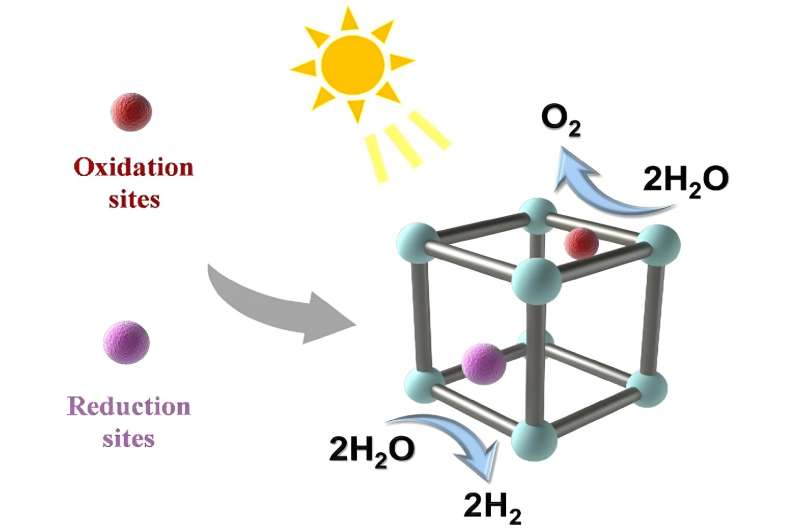
The authors also laid out some of the most promising leads for improvement of use of MOFs for photocatalytic water splitting, in particular the development of MOFs with dual active sites—ones for both parts of the water splitting chemical reaction—the "hydrogen evolution reaction" and the "oxygen evolution reaction."
Dual active sites can provide more active sites for the adsorption (the process where the molecules of a substance attach themselves to the surface of another substance) and activation of water molecules. The paper proposes that the dual active sites can be achieved by introducing two different types of metal ions or organic linkers into the MOF structure, or by introducing a co-catalyst (material that is used in conjunction with a photocatalyst to enhance its performance, in this case such as a noble metal) onto the MOF surface.
However, the paper also notes that the design and synthesis of MOFs with dual active sites remains still a challenging task. This is because it requires precise control over the MOF structure and composition.
In addition, the introduction of two different types of metal ions or organic linkers into the MOF structure, or the introduction of a co-catalyst onto the MOF surface, can affect the stability and activity of the MOF. Advancing the development of MOFs with dual active sites requires careful consideration of factors such as the size and shape of the MOF crystals, the authors conclude, as well as the arrangement of atoms surrounding the MOF's central metal ion, and the interactions between the MOF and the co-catalyst.
Lastly, the paper suggests that the performance of MOFs with dual active sites can be affected by factors such as the loading amount and distribution of the co-catalyst, the surface area and porosity of the MOF, and the reaction conditions.
More information: Yang An et al, Metal–organic framework-based materials for photocatalytic overall water splitting: Status and prospects, Polyoxometalates (2023). DOI: 10.26599/POM.2023.9140030
Provided by Tsinghua University Press