This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Hot chemistry quickly transforms aromatic molecules into harmful aerosols: Study
Joint research groups at Tampere University, University of Helsinki, Lund University and Pi-Numerics, Salzburg, have established key early steps in the conversion of aromatic molecules, a major constituent of traffic and other urban volatile emissions, into aerosol. Published in Nature Communications, their findings increase understanding of the chemical processes that degrade urban air quality and influence climate change.
Many aromatic molecules are carcinogenic and have negative impacts on health. Their primary source is exhaust fumes from motor vehicles. Aromatics can form aerosol particles when they collide in the atmosphere with the hydroxyl radical, a molecule colloquially dubbed "atmospheric detergent" due to its acute propensity to react chemically.
When breathed in, aerosol particles can lead to a myriad of chronic health issues and even death. These particles also affect Earth's climate by reflecting sun light and increasing the formation of clouds.
Despite their importance to the urban environment, details of the reaction processes that form aerosol from aromatics have until now remained unresolved.
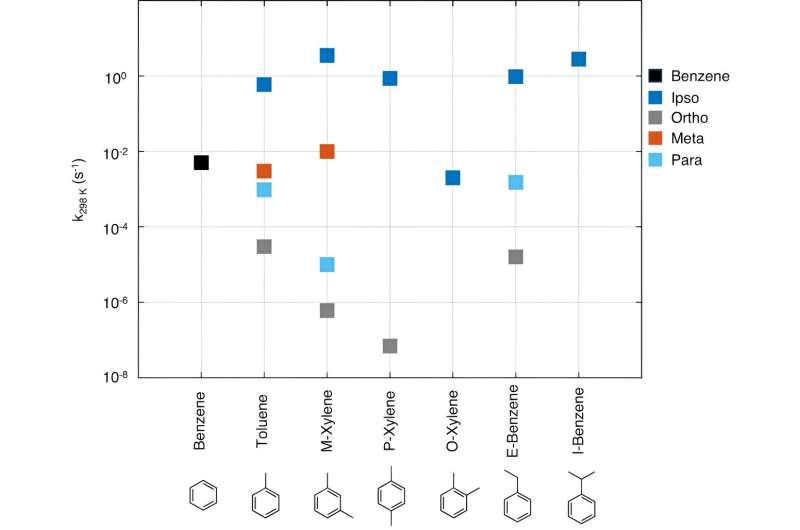
The group of researchers used a combination of quantum mechanics, targeted experiments, and modeling to establish the early steps in the reaction process of toluene, one of the most abundant aromatic molecules.
"We found out that a reaction product that was previously thought to be stable is in fact transient and converts to new hot molecules. These molecules have residual energy that makes subsequent chemistry fast and promptly lead to aerosol precursor products," says Siddharth Iyer, Postdoctoral Research Fellow of Aerosol Physics at Tampere University.
"This result bridges the gap between theory and observation and provides better understanding of the chemistry of aerosol formation in urban environments."
More information: Siddharth Iyer et al, Molecular rearrangement of bicyclic peroxy radicals is a key route to aerosol from aromatics, Nature Communications (2023). DOI: 10.1038/s41467-023-40675-2
Journal information: Nature Communications
Provided by Tampere University