This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
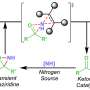
Electrochemical flow aziridination of terpenes
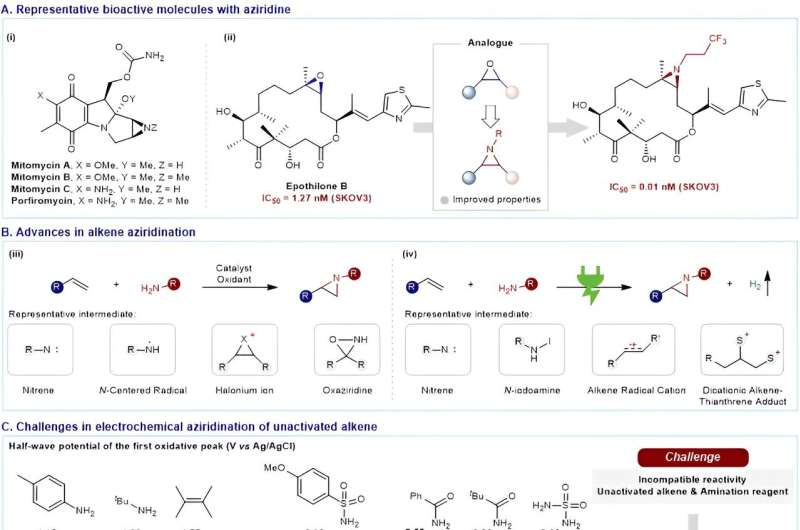
Due to the inherent physiological properties of nitrogen-containing heterocyclic moieties, the construction of nitrogen-containing compounds has emerged as one of the central issues in contemporary synthetic chemistry over recent decades. Among these nitrogen-containing compounds, aziridines displayed distinct biological activities.
As analogs of epoxide, the metabolites of olefins, aziridines derived from bioactive molecules may manifest pharmacological activities. As a consequence, there has been increased interest in developing innovative synthetic methodologies for the production of aziridines from bioactive natural products.
Presently, four canonical strategies have been established for olefin aziridination: nitrene, nitrogen-centered radical, halonium ion and oxaziridine methods. These reactions frequently necessitate pre-functionalized amination reagents, stoichiometric oxidants and transition-metal catalysts, which compromise their atom economy and potential further applications.
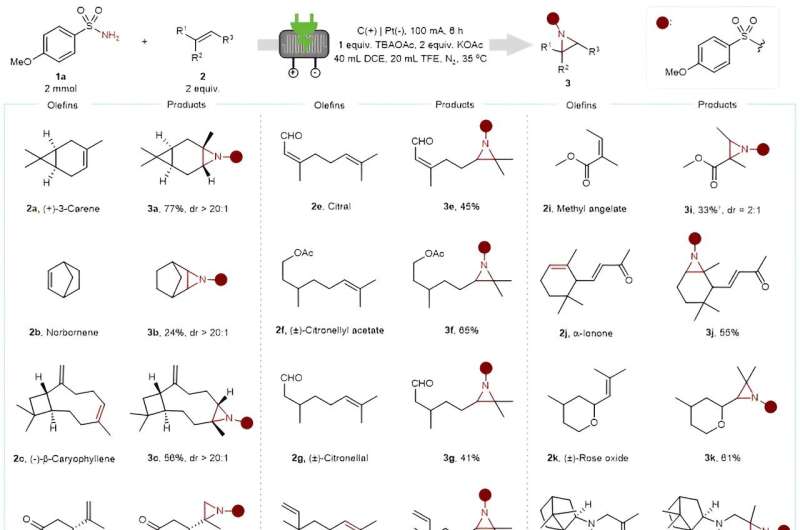
In the past two decades, the resurgence of electrochemistry has yielded environmentally friendly and sustainable approaches for olefin aziridination. However, these electrochemical methods for olefin aziridination have been constrained to specific substrates, predominantly electron-rich olefins, presenting challenges in natural product modification. The most significant hurdle lies in is reconciling the incompatible reactivity of unactivated olefins and amination reagents.
Drawing from wellspring of previous research, Aiwen Lei (Institute of Advanced Studies, Wuhan University) and co-workers hypothesized that the simultaneous oxidation of alkenes and amination reagents might provide a pathway for achieving olefin aziridination through radical/radical cation cross-coupling.
The radical/radical cation species cross-coupled with another one since they are generated close to the anode, thereby circumventing the need of catalyst and extra-oxidants. Furthermore, the utilization of an electrochemical flow cell could pave the way for a scalable approach for olefin aziridination.
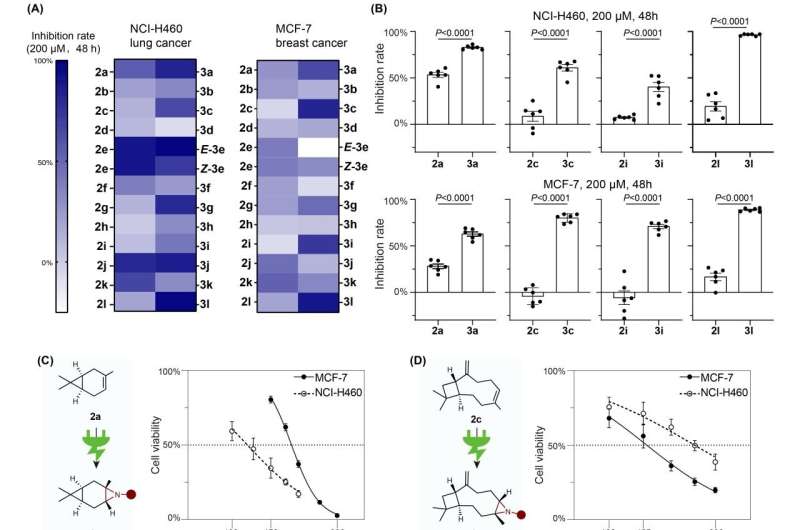
This method demonstrates impressive compatibility with a variety of bioactive molecules with more than 15 kinds of natural products and drug derivatives. Investigation into anticancer activity and synthetic transformation of aziridines bolster the potential of this electro-oxidative reaction in the organic synthesis and medical discovery, thus offering a novel synthetic strategy for olefin aziridination and furnishing an alternative approach to discovery of new drug candidates.
The research is published in the journal National Science Review.
More information: Shengchun Wang et al, Electrochemical flow aziridination of unactivated alkenes, National Science Review (2023). DOI: 10.1093/nsr/nwad187
Provided by Science China Press