August 16, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers characterize influenza adaptation to human epithelial cells, with surprising results
The 1968 influenza pandemic was caused by the H3N2 flu strain and killed between 1 and 4 million people globally. For the sake of comparison, the WHO estimates that around 3 million people died of COVID-related illness in the year 2020.
The initial outbreak of the 1968 flu pandemic appeared in Hong Kong on July 13 and may have spread from mainland China. Lacking the extreme morbidity of the 1918 flu pandemic, the 1968 outbreak is seldom recalled in the media, but the virus continues to circulate with the annual flu cycle and in years when it is the predominant virus, hospitalizations rise. H3N2 presents a particular danger for elderly people.
H3N2 descended from H2N2 through antigenic shift, a process by which a new virus type results from the combination of genes from multiple subtypes. In contrast to the slower process of antigenic drift, shifts can be abrupt and tend to result in major changes like zoonotic transfer.
Scientists previously worked from a paradigm in which the H3N2 virus rapidly acquired human-specific receptor-binding specificity that resulted in a pandemic.
But researchers from Utrecht University and the University of Copenhagen now report that the H3N2 influenza A virus gradually adapted to high-affinity human receptor binding and entry specificity after the start of the 1968 pandemic. The onset of pandemic was instead driven by initially low-affinity interactions of the virus with human epithelial cells—in essence, viral capsids roll across the surface of human cells until they achieve simultaneous low-affinity interactions with a range of receptors, resulting in tight heteromultivalent binding. Only later in the pandemic did the virus evolve high affinity to a specific receptor.
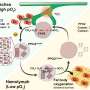
Affinity is the binding strength of interactions between glycoproteins on the surface of the virus—in this case, hemagglutinin—and sialic acid receptors on a human epithelial cell. It is technically difficult to study how a pathogen's range of affinities determines binding selectivity and virus motility. In a paper published in the Proceedings of the National Academy of Sciences, the researchers present a biolayer interferometry assay they performed to analyze the evolution of receptor-binding kinetics at the point of host-switching.
After jumping from avian hosts to humans via weak binding interactions, H3N2 ultimately switched from binding avian sialic acid receptors to structurally different human sialic receptors. The analysis revealed that the virus-binding kinetics of H3N2 evolved from 1968 to 1979 from low mixed specificity (low affinity to a variety of human receptors simultaneously) to high specificity to a single receptor, the human-type Siaα2-6-linked sialic acid receptor. But this evolution continued in a surprising direction; the researchers were surprised to find a decline in selectivity after 1992.
Among the study's findings, it establishes for the first time that neither high selectivity nor high affinity for human-type receptors are necessary for efficient viral spread. They speculate that receptor diversity on epithelial cells can be exploited by a virus carrying low-affinity glycoproteins, thus enhancing receptor-binding plasticity, expanding the paths for antigenic change.
In conclusion, the authors write, "Opposing the canonical view, preferential binding to human-type [sialic acid] receptors evolved slowly for human H3N2 strains and was not maintained over time. Systematic analysis of kinetic-binding parameters will provide a basis for a detailed understanding of the enigmatic HA/NA balance and of the requirements for heteromultivalent binding and its potential role in zoonotic transmission."
More information: Mengying Liu et al, H3N2 influenza A virus gradually adapts to human-type receptor binding and entry specificity after the start of the 1968 pandemic, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2304992120
Journal information: Proceedings of the National Academy of Sciences
© 2023 Science X Network