This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Biochemist unravels the secrets of a novel DNA enzyme linked to infertility and certain cancers
Scientific research requires patience. The rewards are not always immediate, and the technology needed does not always exist. Michael A. Trakselis, Ph.D., professor and director of graduate affairs for the Department of Chemistry and Biochemistry at Baylor University, understands this.
For 15 years, Trakselis has been interested in the MCM8/9 enzyme. Part of the minichromosome maintenance protein (MCM) family, MCM8/9 is directly linked to ovarian insufficiency, infertility and cancers including ovarian, testicular and colon cancers, yet little is known about how this enzyme works and its connection to disease. There was no understanding of the relationship between the structure and function of the enzyme.
The research—Activity, substrate preference, and structure of HsMCM8/9 helicase—was published in the journal Nucleic Acids Research. With this new information, researchers in the Trakselis Laboratory and elsewhere have a roadmap to better understand the function of this enzyme complex and how mutations may be connected to disease.
"Mutations in MCM8 or MCM9 can cause infertility and cancer; yet, we don't know why we get certain diseases when there are mutations in this protein," Trakselis said. "Having the structure might help us figure out some of that."
Before that could happen, Trakselis had to overcome two barriers: technology and a stable protein solution needed to conduct the research.
Until recently, the technology needed to understand MCM8/9 was not readily available. The Single-particle cryo-Transmission Electron Microscope (cryo-EM) is a highly sensitive and expensive microscope that has only been available for use for about eight years.
"It's a massive instrument, but it works to image really tiny things," Trakselis said.
The cryo-EM helps visualize and solve what the structure looks like by shooting electron beams into the material. "Based on how the beams are reflected or refracted, you can start to build a structure."
On this research, collaborating with researchers from Rice University and the University of Texas Health Science System in Houston, Dr. Trakselis was able access a cryo-EM.
The next barrier was creating a stable and pure protein solution. An impure or unstable sample would prevent the cryo-EM from revealing any precise structural information on the MCM8/9 complex. Trakselis and his team tried several approaches to expressing and purifying various constructs of MCM8/9, but they were of low purity and solubility. After much trial and error, the team ultimately tried insect cells for expression of this human protein, which finally resulted in stable pure protein that allowed cryo-EM imaging of the MCM8/9 complex.
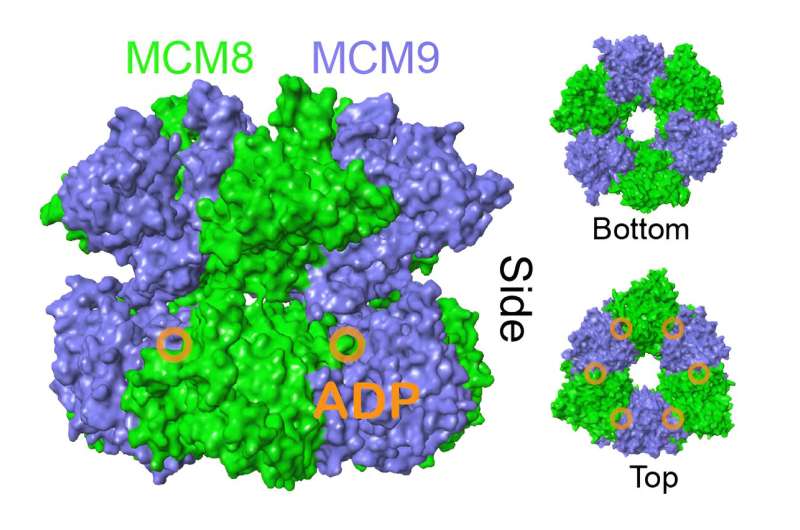
With the barriers removed, Trakselis and his team were able to unravel the structure of MCM8/9 bound to its substrate product, ADP.
For Trakselis this is only the beginning. There are more questions to be answered.
"We described the basic structure-function relationship, but we don't know what it does in the cellular level and in what context," Trakselis said.
More information: David R McKinzey et al, Activity, substrate preference and structure of the HsMCM8/9 helicase, Nucleic Acids Research (2023). DOI: 10.1093/nar/gkad508
Journal information: Nucleic Acids Research
Provided by Baylor University