This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Animal testing under REACH: Bringing numbers into the debate
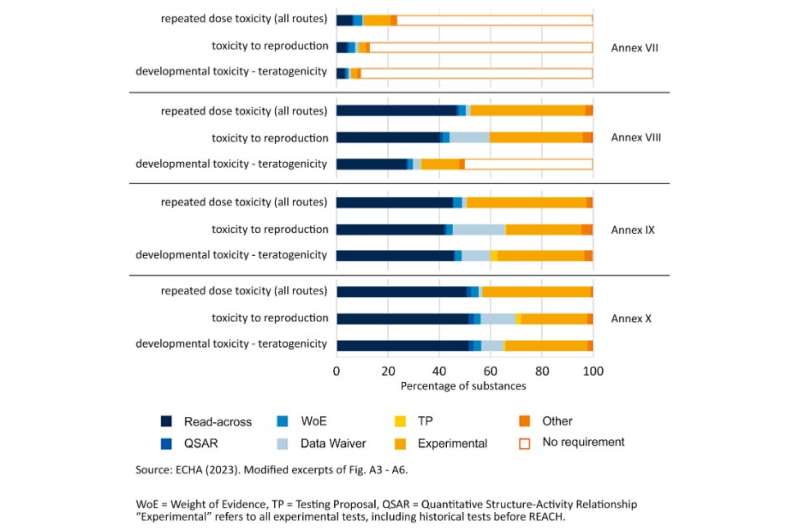
Sixteen years ago, the REACH chemical regulation came into force across Europe. REACH obliges the chemical industry to identify the health risks of all chemicals used in their products. The downside of REACH is that this hazard assessment requires a large number of animal tests. Just how many was not clear until now.
The "Center for Alternatives to Animal Testing" (CAAT) based in Baltimore and at the University of Konstanz now wants to bring numbers into the REACH debate. In two current studies, based on data from the European Chemicals Agency (ECHA), the researchers show that so far around 4.2 million animals have been used for hazard assessment under REACH (of which 1.3 million animals are in ongoing studies). An additional 3.5 to 6.9 million animal tests are expected due to the revision of REACH in 2022. Their studies have been published in the journal ALTEX.
Animal-free, alternative test methods were relatively rarely used. What is known as read-across methods (prediction of toxicity from comparison with structurally similar, already tested chemicals) were rejected in 75% of cases.
Animal-free alternative methods
The researchers from Konstanz and Baltimore advocate the use of animal-free alternative methods (New Approach Methodologies, NAMs). "Some of these new methods are not only suitable for large-scale chemical screenings, but also provide more meaningful results than animal testing, as the chemicals are tested on human cells—naturally in a petri dish," explains Thomas Hartung, Director of the Center for Alternatives to Animal Testing (CAAT) and professor at the University of Konstanz.
"Animal-free alternative methods are available for an increasing range of test purposes. The goal must be to adapt the legislation to the current state of scientific knowledge," demands Marcel Leist, professor of in-vitro-toxicology at the University of Konstanz and co-director of the Center for Alternatives to Animal Testing Europe. The CAAT researchers emphasize the importance of bringing scientists, authorities and industry to the same table to advance the introduction of alternative methods.
More information: Costanza Rovida, REACH out-numbered! The future of REACH and animal numbers, ALTEX (2023). DOI: 10.14573/altex.2307121
Jean Knight, 4.2 million and counting… The animal toll for REACH systemic toxicity studies, ALTEX (2023). DOI: 10.14573/altex.2303201
Provided by University of Konstanz