June 23, 2023 report
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
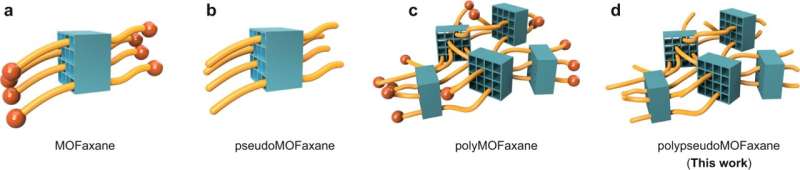
A new class of interlocking supramolecular systems: MOFaxanes
A team of chemists and material scientists at the University of Tokyo has developed a new class of interlocking supramolecular systems by combining metal-organic frameworks with rotaxanes. In their study, reported in the journal Nature Communications, the group combined the two structures and found possible uses for the results.
Metal-organic frameworks (MOFs) are compounds made using metal ions in such a way as to create one-, two-, or three-dimensional structures. The resultant ligands are known in the chemistry world as linkers or struts. They are typically used to make products such as sensing equipment, machines that store energy or those that separate and purify liquids. They have also been used for biological imaging and drug delivery.
Rotaxanes are molecular structures that are interlocked in dumbbell shapes. They are created by threading cyclic molecules into other molecules and then applying end caps. They are typically used as molecular switches in electronics devices, and sometimes as shuttles. In this new effort, the research team developed a way to connect the two types of compounds to create new kinds of interlocking structures.
The researchers noted that MOFs have nanometer-sized pores, which are used to coordinate interactions with other molecular structures, a property that has made them useful in a wide variety of applications. They also noted that rotaxanes are molecular machines. Looking at the two structures gave them the idea to connect them.
They found that doing so was relatively easy. They simply threaded rotaxane polymer chains through the pores in a MOF made using copper. This led to the creation of what they call MOFaxanes. They noted that by using extra-long polymers, they were able to reverse the types of relationships typically seen with MOFs and their "guests," allowing for greater differences in size between the two than is typical. They also note that because MOFs are so tunable, the range of creatable MOFaxane structures types and sizes should be virtually unlimited.
More information: Tomoya Iizuka et al, An approach to MOFaxanes by threading ultralong polymers through metal–organic framework microcrystals, Nature Communications (2023). DOI: 10.1038/s41467-023-38835-5
Journal information: Nature Communications
© 2023 Science X Network