This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists address global crop growing challenge
A study led by University of Liverpool scientists has revealed a new way to improve crop growth, meeting a significant challenge to increase crop productivity in a changing climate with a growing population.
With global levels of carbon dioxide (CO2) rising and the population set to reach almost 10 billion by 2050, Professor Luning Liu's team of researchers used synthetic biology and plant engineering techniques to improve photosynthesis, creating a template that can be used on a mass scale.
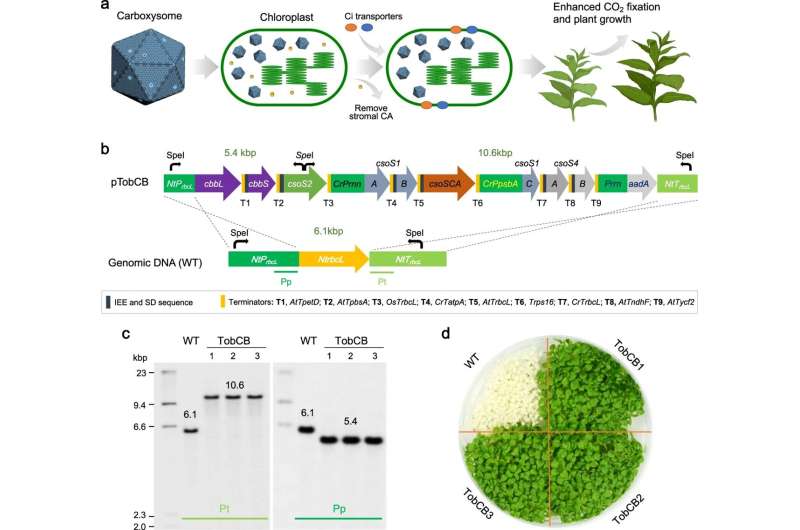
Photosynthesis is the process by which plants use atmospheric CO2 to create nutrients, which are crucial for growth and the global ecosystem. The newly published paper details how the team of scientists have improved Rubisco, a key enzyme present in photosynthesis that converts CO2 into energy. Usually Rubisco is inefficient and limits photosynthesis in major crops. However, many microorganisms including bacteria have evolved efficient systems, named "CO2-concentrating mechanisms," to improve Rubisco.
Inspired by nature, the team has successfully engineered a catalytically faster Rubisco taken from bacteria, into tobacco plant cells that undertake photosynthesis to support plant growth. The new method improves the Rubisco's stability and ability to convert CO2 into energy, allowing plants to further thrive. The changes to the enzyme also potentially increase the plants ability to absorb CO2, helping to support the global effort to address climate change.
Professor Luning Liu, Department of Biochemistry and Systems Biology, University of Liverpool said, "We are extremely excited with this breakthrough. Overall, our findings provide proof-of-concept for a route to improving crop development and production that can withstand changing climates and meet the growing food requirements of the world's expanding population."
This latest study follows the team's recent attempt to engineer the faster Rubisco from bacteria to support plant growth.
The research is published in the journal Nature Communications.
More information: Yongjun Lin, Engineering α-carboxysomes into plant chloroplasts to support autotrophic photosynthesis, Nature Communications (2023). DOI: 10.1038/s41467-023-37490-0. www.nature.com/articles/s41467-023-37490-0
Journal information: Nature Communications
Provided by University of Liverpool