This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Comparing ice and water in the quenching of heat in metal

Associate Professor Jonathan Boreyko leads a team at Virginia Tech that has built a strong portfolio of work with ice and water, exploring the possibilities for de-icing planes, building novel water harvesting devices, and creating snow globes out of bubbles. This familiarity with water has given the team a strong sense of its behavior in different states, leading to a new project that shows how ice quenches heat in comparison to water. The findings were published in the journal Chem on April 14.
Mojtaba Edalatpour and master's student Camryn Colón carried out this project. They investigated methods of quenching heat from metal, a critical step in applications such as metallurgy and firefighting. Both instances require speed. Metallurgists need to rapidly drop the temperature of a forged piece to achieve specific material properties, while firefighters work to stop destruction of property as quickly as possible. Quenching with water is only effective beneath a critical temperature—any higher and the water levitates on its own vapor and can no longer boil the heat away.
Boreyko's team wanted to see if using ice, rather than water, could bypass the levitation issue to enable the quenching of ultra-hot surfaces.
Measuring, heating, and measuring again
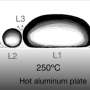
To conduct this research, Edalatpour and Colón heated an aluminum stage and measured the cooling rate of water versus ice. To ensure a direct comparison, they released the same amount of water and ice onto the surface after it was heated to a desired temperature.
When the initial surface temperature of the stage was between 100°C and 300°C, both the water and the ice successfully quenched the surface below 100°C. The ice, however, achieved that result in half the time. At higher initial temperatures—300°C to 500°C—only quenching with ice was successful. Heat transfer with ice was more than 100 times more effective than with liquid water at these high temperatures.
What was the difference? The properties of water prevent it from hitting the sweet spot for removing heat.
That sweet spot is boiling, because the steam escaping in bubbles most efficiently carries the heat away. Because water easily levitates on its vapor at high temperatures, it becomes insulated from the surface and the boiling never occurs. Ice behaves differently. When dropped onto a hot surface, ice absorbs much of the heat as it melts. This reduces the amount of heat available for producing vapor bubbles, preventing the levitation problem. In other words, the meltwater boils at a slower pace compared to pure water, thus helping to maintain boiling at high temperatures.
Boreyko compared the unusual liquid behavior with worker productivity.
"Think about a workaholic who is always focusing on their job," he said. "They start off hyper-productive but quickly burn out and become ineffective. It turns out that water is the same way when exposed to ultra-high temperature surfaces: It is so focused and productive at boiling water into vapor that it experiences 'burnout,' which is the scientific term for levitation and the catastrophic failure in cooling that results. So ice is like the slow and steady tortoise that wins in the long run. It doesn't make vapor bubbles very well, but this allows it to keep boiling and avoid levitation when things get heated."
The frozen path continues
The group's hypothesis of using ice for quenching followed its recent discovery that ice does not levitate and lose its boiling capability until 550°C, compared to 150°C for water. Based on those findings, Boreyko's team began several new projects applying its principles. This heat transfer is the first outgrowth to be published.
Colón's follow-up work includes measuring the cooling performance of ice when the surface is fixed at a constant temperature rather than being allowed to cool down.
"When you have a constant temperature, you can measure the steady-state heat flux, which would allow us to directly compare the heat transfer of ice versus state-of-the-art boilers," said Colón.
The team is also brainstorming how to implement a practical ice quenching system.
"It remains to be seen exactly how to implement three-phase heat transfer for real life applications, but we're excited to figure it out over the next several years," said Boreyko. "It might involve making spray nozzles that are able to eject ice particles instead of water, or perhaps it will look more like releasing a pre-formed block of ice onto an overheated surface. There's a lot more to figure out before this becomes an on-the-shelf technology."
More information: Jonathan R. Boreyko, Ice quenching for sustained nucleate boiling at large superheats, Chem (2023). DOI: 10.1016/j.chempr.2023.03.010. www.cell.com/chem/fulltext/S2451-9294(23)00129-8
Journal information: Chem
Provided by Virginia Tech