This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Fiery response: 'Siglec-14' receptors on human macrophages detect carbon nanotubes and provoke inflammation
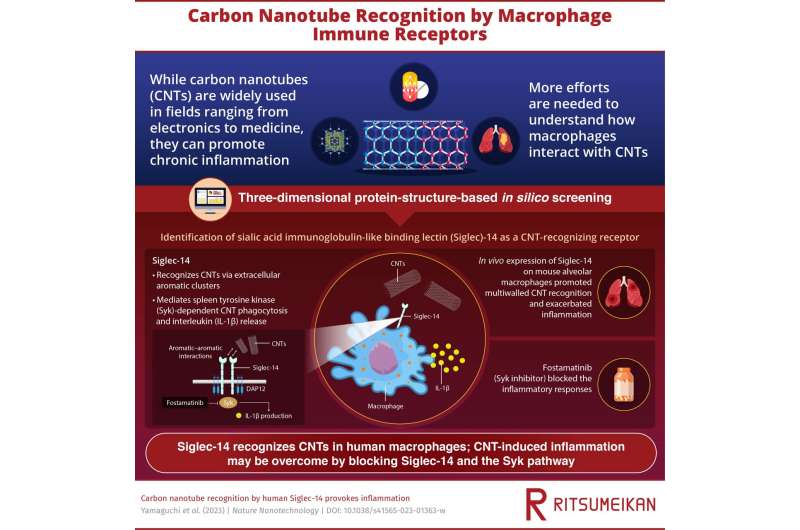
Carbon nanotubes (CNTs) have become a mainstay of the field of nanotechnology. Finding innovative applications across materials science, electronics, and medicine, CNTs have garnered a lot of attention from researchers in recent years. However, the International Chemical Secretariat (ChemSec) has moved to flag CNTs on the "Substitute It Now!" database of chemicals likely to be restricted for use. In fact, due to their persistence in nature and potential toxicity to humans, ChemSec has proposed that adequate assessments of CNTs' risk to human health are urgently needed.
Following their entry into the body, and much like asbestos, CNTs are targeted by the immune system and preferentially engulfed by macrophages. The ensuing inflammatory response—involving inflammasome and interleukin-1β (IL-1β) secretion—can progress to chronic inflammation, fibrosis, and even mesothelioma, as seen in rodent models. Unfortunately, how human macrophages recognize CNTs has remained a mystery.
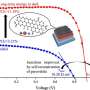
Now, a team of researchers in Japan have found that extracellular receptors, namely, sialic acid immunoglobulin-like binding lectin (Siglec)-5 and -14, are involved in CNT recognition by human macrophages. The team, which included Professor Masafumi Nakayama from Ritsumeikan University, built upon their previous work that confirmed how mouse T-cell mucin immunoglobulin 4 (Tim4) receptors bound to CNTs via an aromatic-aromatic interface.
The findings from this study have been published in Nature Nanotechnology. "We hypothesized that aromatic clusters were essential to CNT recognition. But at the time, it wasn't evident that Tim4 receptors had a role in CNT engulfment by human macrophages," says Prof. Nakayama of his motivation for the study.
The group used three-dimensional protein-structure-based in silico screening and ectopic in vivo expression of Siglec-14 receptors to validate how the aromatic rings facilitated the Siglec-CNT interaction. Via molecular dynamics simulations, the team discovered the interaction of aromatic residues on the extracellular loop of Siglec-5 and CNTs. On the other hand, they found that Siglec-14 initiated the spleen tyrosine kinase (Syk) pathway-dependent phagocytosis of CNTs and the release of IL-1β by macrophages. The detection of CNTs was enhanced in mouse macrophages expressing human Siglec-14, and importantly, the team noticed that this interaction greatly exacerbated pulmonary inflammation.
Lastly, the drug fostamatinib, which is a Syk inhibitor, was seen to block the Siglec-14-mediated proinflammatory signal cascade. "This is an encouraging result as this drug may overcome CNT-induced inflammation," says Prof. Nakayama as he elaborates on the significance of the teams' work.
While the team is excited about their breakthrough, they are cautious to note that these findings do not mean CNTs have asbestos-like toxicity. Estimating the toxicity of CNTs would depend on a host of other variables: the route of exposure, the CNT dose received, and the size and shapes of the CNTs involved. At this stage, more detailed studies are required to accurately gauge the risk posed to humans.
However, Prof. Nakayama takes pride in knowing that his team have laid the groundwork to help develop safer CNTs. He concludes, "Even if CNTs are likely to cause inflammatory diseases, our findings will help develop novel therapies, like anti-Siglec-14 monoclonal antibodies and fostamatinib, to prevent such conditions."
More information: Kota Kasahara, Carbon nanotube recognition by human Siglec-14 provokes inflammation, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01363-w. www.nature.com/articles/s41565-023-01363-w
Journal information: Nature Nanotechnology
Provided by Ritsumeikan University