This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Label-free superior contrast with c-band ultra-violet extinction microscopy
A new purpose-built microscope has been developed that uses the complex properties of UV light to improve image resolution and contrast. Most cells are transparent at visible wavelengths, which means the imaginary component of their complex refractive index, also known as extinction coefficient, is close to zero.
In the c-band of the ultra-violet region, however, the extinction coefficient is naturally much higher than at visible wavelengths. Using a new UV-compatible microscope with relevant image processing results in excellently contrasted, high-resolution, label-free microscopy of conventionally invisible features of biological samples.
The use of high-resolution bright-field microscopy is essential for cellular-scale biological research, but this technique has limitations due to low image contrast. Using dyes on the sample can result in excellent contrast, but this requires additional time and effort and can alter the natural structure or function of the cell. Thus, intrinsic contrast methods are preferable—ideally ones that provide quantitative information.
A new candidate for such a method involves the use of c-band ultra-violet (UVC) light, which both improves the image contrast and provides inherent depth sectioning in biological samples due to UVC's lower penetration depth, greater absorption, and higher specificity. This approach provides the possibility of visualizing small structures inside cells that are close to, but above, the resolution limit and are often not detected with traditional bright-field microscopy.
Using computational quantitative phase microscopy (QPM), this new method can extract and separate the numerically real and imaginary components of the image of a sample. Because a cell's extinction coefficient, which is related to the imaginary phase information, is typically very small and contributes little to image contrast, conventional QPM usually neglects this component.
However, since the extinction coefficient is typically much higher for UVC than for visible light, UVC QPM adds a new layer of information in the form of highly contrasted, quantitative extinction coefficient maps as a complementary measurement to traditional quantitative phase maps.
The key to this method is the wavelength-dependent change in absorption of biological materials that contain proteins, lipids, or nucleic acids. These show a distinctive "hockey stick" shape when plotting wavelength versus absorption: absorption increases as wavelength decreases, with a sharp increase right where the UVC band begins. This extra absorption makes UVC light a useful tool to generate higher contrast in cell imaging, without needing to add artificial dyes.
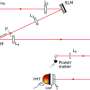
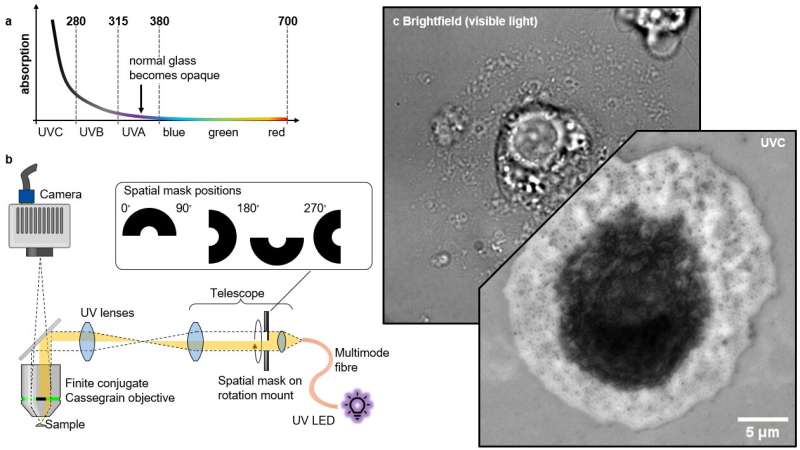
When trying to implement the method in practice, the authors faced several challenges in developing the technique. Firstly, standard microscope lenses typically do not transmit UVC light, as the glass they are made from is typically UV-opaque. Secondly, glass refractive indices exhibit stronger dispersion at short wavelengths, making chromatic correction in the UVC regime challenging.
To overcome these challenges, the authors used a special mirror-based microscope objective, specifically a high numerical aperture Cassegrain-type objective in finite conjugation, to avoid chromatic aberrations and to improve transmission in UVC. They also employed the recently-developed reflection quantitative differential phase contrast (qDPC) algorithm to retrieve complex refractive index distributions. This algorithm requires a set of obliquely-illuminated intensity images for retrieval of the imaginary phase information, which the authors achieved by including a UV-transmissive fused silica lens relay with a rotatable semi-circular amplitude mask.
The mask blocks half the pupil and shields the objective's secondary mirror from on-axis illumination, preventing stray light from obscuring the light of interest coming from the sample. Additionally, other design choices helped keep the light homogeneous in the field of view while reducing the light exposure to the sample outside of the imaging area, e.g. conjugating the output facet of a large diameter solarization-resistant multi-mode fiber to the image plane.
The authors also chose a UVC LED with a narrow (10 nm) bandwidth to minimize chromatic effects and ensure a well-defined operating wavelength for the retrieval algorithm. The resulting microscope should theoretically be able to achieve 212 nm in resolution, and in practice the authors achieve nearly that, with a resolution of ~215 nm measured in images of standard cell lines after qDPC processing.
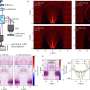
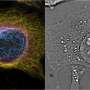
With their new UV microscope, the authors studied liver sinusoidal endothelial cells (LSECs), which are thin, fluid-filtration cells in the liver which contain small holes called fenestrations. These fenestrations are challenging to visualize using conventional light microscopy because of their small size (50-300nm).
It is noteworthy that LSECs are involved in the body's blood filtration system, and understanding their structure and function is crucial in disease research and drug discovery.
The ability to visualize and study the sub-diffraction limit sized fenestrations of LSECs with this new UV microscope is an exciting development that could lead to a better understanding of the role of these cells in the human body. The authors compared their UV microscope to other microscopy techniques, including brightfield, differential interference contrast (DIC) microscopy, and a commercial holotomography system, and found that the UV microscope provided superior contrast and was the only technique that allowed them to visualize individual fenestrations close to the diffraction limit.
This highlights the challenge that label-free methods face when imaging thin and nanoscale biological specimens that do not scatter significantly. By relying on absorption, the UVC microscope overcame this problem and could exploit the significant extinction coefficients in this wavelength regime to obtain better contrasted quantitative images from a thin and nanoscale biological sample.
Using their UV microscope, the authors were able to segment individual potential fenestrations clusters and determine their respective extinction coefficients. They found that on average the extinction coefficients of fenestrations were higher than the average extinction coefficient of the plasma membrane and hypothesized that the density of cytoskeletal protein around the fenestrations leads to higher absorption despite the missing biological material in the "holes" of the fenestrations.
The authors also tried to use deep UV illumination to generate autofluorescence, but they detected only very small amounts of signal in the LSECs' fenestration clusters and just a diffuse glow from the nuclear region, which was insufficient for high-resolution images. Overall, the authors demonstrated the capabilities of their UV microscope for studying LSECs and visualizing sub-diffraction limit-sized fenestrations, which could have important implications for understanding the liver's filtration system and related diseases.
In summary, the use of UVC light absorption in high-resolution bright-field microscopy offers significant advantages over traditional staining methods. This technique provides a complementary measurement to quantitative phase maps, offering a more accurate and efficient way of conducting research and scientific discovery without labeling, thus preserving the natural structure of the cell.
The paper is published in the journal Light: Science & Applications.
More information: Florian Ströhl et al, Label-free superior contrast with c-band ultra-violet extinction microscopy, Light: Science & Applications (2023). DOI: 10.1038/s41377-023-01105-6
Journal information: Light: Science & Applications
Provided by Chinese Academy of Sciences