This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study describes the structural and functional effects of several mutations on the androgen receptor
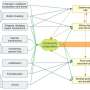
![High-resolution crystal structures of AR-LBD dimer interface mutants reveal local and long-range conformational changes.(A) Superimposition of current crystal structures of mutant AR-LBD on WT monomeric (1T7T) and dimeric (5JJM) forms of the domain. Secondary structure elements identical in all structures are depicted as a gray cartoon, with DHT as teal spheres and the AF-2-bound peptide (from 5JJM) in red. Large main- and/or side chain conformational changes cluster in four areas (B to E), highlighted by the major secondary structure elements (coral sticks). (B) Dimer interface core lined by H5 and H7, which, in addition to the studied point mutations [Phe755 and Val758 (H5), Tyr764 (S1), and Gln799 (H7)] features mostly residues with nonpolar/aromatic side chains, along with the positively charged Arg761. (C) The more distal part of the dimer interface formed by H3 and L1-3 [Tyr774 and His777 from (B) are shown for orientation]. In this area, several polar residues exhibit noticeable conformational changes. (D) BF-3 pocket, where multiple residues exhibit conformational changes, most notably those from H9. (E) AF-2 pocket, where in addition to the charge-clamp residues, Lys721 and Glu894, known to stabilize bound coregulators at this interaction site, the side chains of both charged (Lys718, Arg727, Lys823, and Glu898) and aliphatic residues (Met735 and Met895) display conformational variability. (F) Sector 1 (teal) comprises 17 residues in and around H5, H7, H8, H9, and H10-11 (Met743, Phe748, Gly751, Arg753, Leu798, Ile800, Thr801, Met808, Leu811, Phe814, Glu838, Ile842, Thr851, Tyr858, Thre861, Lys862, and Leu864), whereas sector 2 (green) features 20 residues mostly from H3, H5, H7, H8, S3, and H10-11 (Arg711, Leu713, Trp719, Ala720, Lys721, Phe726, Leu729, Gln734, Tyr739, Trp742, Gly744, Met746, Ala749, Trp752, Ser754, Leu791, Lys809, Leu813, Asp820, and Arg856). Last, three residues (Gly725 and Ile738 at the AF-2 groove and Phe805 at H7) belong to both sectors (yellow). Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade2175 Study describes the structural and functional effects of several mutations on the androgen receptor](https://scx1.b-cdn.net/csz/news/800a/2023/study-describes-the-st.jpg)
The androgen receptor is a key transcriptional factor for the proper sex development—especially in males—and the physiological balance of all the tissues that express this receptor. The androgen receptor is involved in several pathologies and syndromes, such as the spinal and bulbar muscular atrophy or androgen insensitivity syndrome, among others, for which there is no specific treatment.
Regarded as the main initial and progression factor in prostate cancer—the second most common malignant disease in men in industrialized countries—this receptor has been, for decades, the main therapeutical target for the treatment against this disease.
Now, a study published in Science Advances describes the structural and functional effects of mutations on the androgen receptor, as well as how these changes lead to the development of prostate cancer. The study is led by lecturer Eva Estébanez-Perpiñá, from the Department of Biochemistry and Molecular Biomedicine of the Faculty of Biology and from the Institute of Biomedicine of the University of Barcelona in collaboration with the experts Pablo Fuentes-Prior, former head of a research group at the Research Institute of Sant Pau (IBB Sant Pau), and Álvaro Aytés, from the Bellvitge Biomedical Research Institute (IDIBELL) and the Catalan Institute of Oncology (ICO).
The study, whose first co-authors are Andrea Alegre and Alba Jiménez (UB-IBUB) and Adrián Martínez (ICO and IDIBELL), includes the participation of the team led by lecturer Jaime Rubio Martínez, from the Faculty of Chemistry and the UB Institute of Theoretical and Computational Chemistry (IQTC), and groups from CSIC and the National Institute of Health and Medical Research in France (INSERM).
Point mutations in the androgen receptor
The human androgen receptor is a key protein in the development and functioning of the prostate in response to male hormones, such as testosterone. Point mutations in the androgen receptor—specifically, one amino acid changing for another—are one of the main mechanisms than can lead to structural and functional alterations in the receptor, which result in the development of diseases.
The results of the study show that the analyzed mutations affect several functional regions of the union domain of the androgen receptor to testosterone. In particular, these are mutations that alter a region of the receptor which is the target for posttranscriptional modifications (that is, modifications in the protein once this is produced).
These types of chemical alterations affect specific amino acids of the androgen receptor and are executed by regulating proteins which are decisive for the proper functioning of the receptor. If this receptor's regulation pathway is altered—such as the case of the presence of mutations described by the team—its function is deregulated and it can be dysfunctional and cause pathologies.
"In our study, we experimentally checked that these mutations deregulate a specific mutation, known as arginine methylation, which is one of the posttranscriptional modifications, due to the structural changes these alterations produce in a functional area of the receptor. Also, we could observe that the deregulation of the androgen receptor methylation involves relevant changes in its function within the cell," the team concludes.
More information: Andrea Alegre-Martí et al, A hotspot for posttranslational modifications on the androgen receptor dimer interface drives pathology and anti-androgen resistance, Science Advances (2023). DOI: 10.1126/sciadv.ade2175
Journal information: Science Advances
Provided by University of Barcelona