January 16, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Understanding the alpha smooth muscle actin–driven foreign body response during wound healing
The foreign body response is a clinically relevant process that can lead to issues with biocompatibility in implanted medical devices due to fibrosis. While the inflammatory nature of the foreign body response is already established, bioengineers still seek to understand underlying fibroblast-dependent mechanisms during wound healing.
In a new report now published in Science Advances, Maria Parlani and a team of scientists in oncology and bioengineering in the U.S., and Netherlands, combined multiphoton microscopy with animal models expressing a modified smooth muscle alpha actin (αSMA) protein to investigate the dynamics of fibroblasts relative to their activation and fibrotic encapsulation of polymer materials.
During the experiments, they noted the invasion of fibroblasts as individual cells that developed a multicellular network with a two-component fibrotic response to display an external cold capsule of smooth muscle cells and a relatively hotter and long-lasting inner alpha smooth muscle actin environment. Parlani and colleagues noted how the recruitment of fibroblasts and the extent of fibrosis inhibited after macrophage depletion. These outcomes implied the co-existence of macrophage-dependent and macrophage-independent mediators. The results highlighted the foreign body response as a conserved and self-organizing process that is partially independent of macrophages; specialized cells involved in enabling an immune response in vivo.
The foreign body response during the process of fibrosis
The foreign body response is an ultimate outcome of inflammation and wound healing after biomaterial implantation in a biological environment. This pathophysiological process has increasingly received clinical attention due to its role in inflammation and fibrotic encapsulation of patients' medical implants that can compromise the long-term integration and functional viability of biological implants. The step-wise process of the foreign body response begins with vascular damage and plasma protein engagement on an implant surface, followed by neutrophilic inflammation, monocyte recruitment that leads to macrophage activation as well as the formation of foreign body giant cells.
Interstitial fibroblasts are central effectors of tissue homeostasis and are central to tissue remodeling and wound healing. The progression of wound healing is a transient fibrotic process in which myofibroblasts undergo elimination via apoptosis. The principles governing fibroblast engagement during the foreign body response have yet to be systematically investigated. The researchers therefore sought to understand the impact of macrophages and materials composition on fibroblast activation and on the outcome of fibrosis.
Fibroblast activation after biomaterial implantation
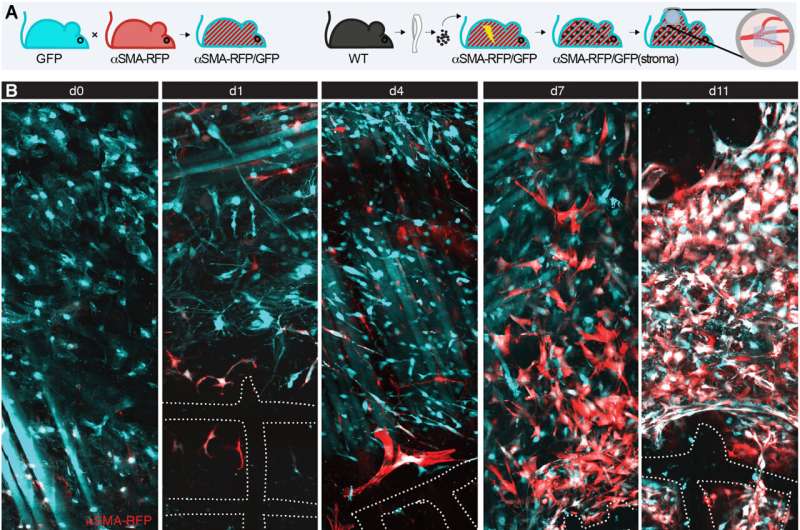
The research team monitored the engagement and fate of fibroblasts during the foreign body response within the deep dermis in a mouse model implanted with a fibrous polymer scaffold. They noted how deeper skin layers of the animal model contained adipocytes, nerves and muscle fibers. The team observed fibroblast activation and alpha smooth muscle actin upregulation within 24-hours of implanting the biomaterial.
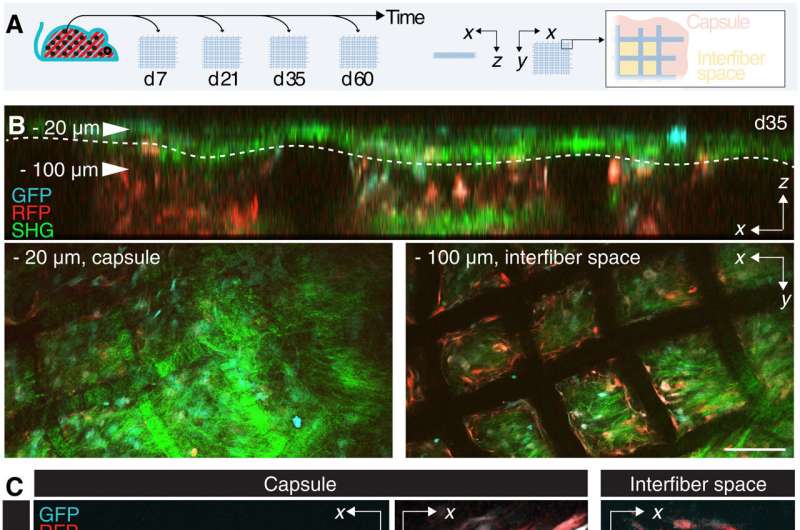
The scientists then identified the impact of alpha smooth muscle actin upregulation in mice implanted with scaffolds at diverse timepoints. They noted two regions of interest: a fibrotic capsule containing fibroblasts and bundled collagen connected to the surrounding interstitial tissue, and an inner core with cells and collagen molecules filling up the space between implants. These outcomes highlighted the foreign body response as a two-compartment process.
Alpha smooth muscle actin (αSMA) fibroblast self-organization and interaction with the implant
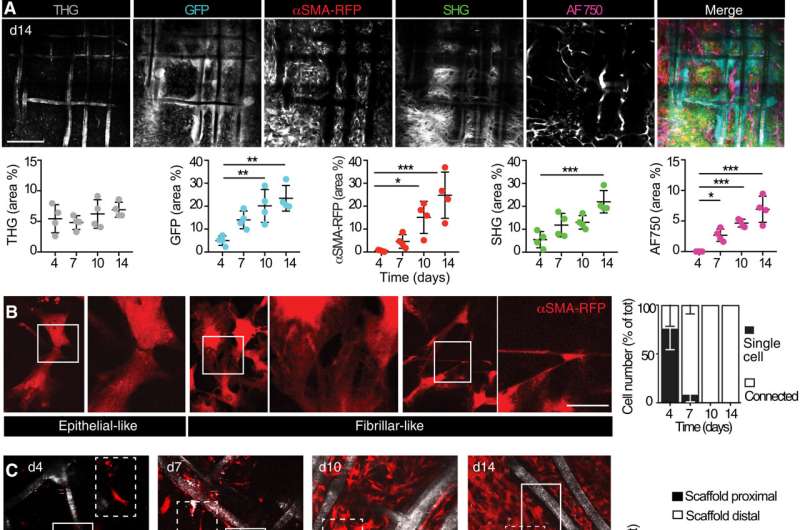
Upon biomaterial implantation, the team gained insights to the organization of alpha smooth muscle actin cells during biomaterial encapsulation by using nonlinear intravital multiphoton microscopy. The αSMA fibroblasts first populated the implant site without directly engaging with the material to establish a multicellular network. This included the process of collagen secretion. The researchers then identified the presence of sub-components that enriched the specialized foreign body giant cells to affect the recruitment process and the positioning of alpha smooth muscle actin after scaffold implantation.
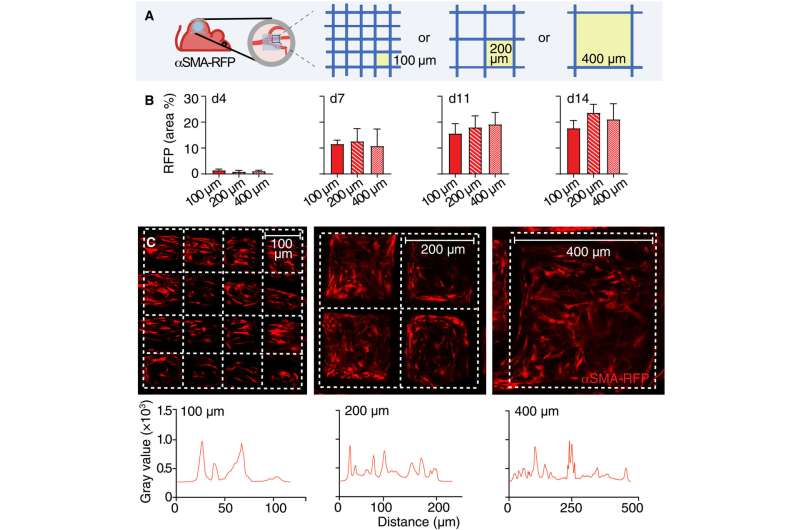
The team noted how the implantation of a polymer scaffold induced recruitment, activation, and redistribution of the αSMA cells. Additionally, since the macrophages and foreign body giant cells are two key regulators of the foreign body response during fibrotic encapsulation of an implant, the researchers reduced the macrophage lineage in scaffold-implanted mice.
They accomplished this by using a chemical that caused apoptosis to ablate macrophages and the giant cells and thereby markedly reduce collagen deposition and fibrotic encapsulation around the implant. While these experiments reduced the recruitment of infiltrating immune cells and decreased the αSMA cell count, they did not impair their activation.
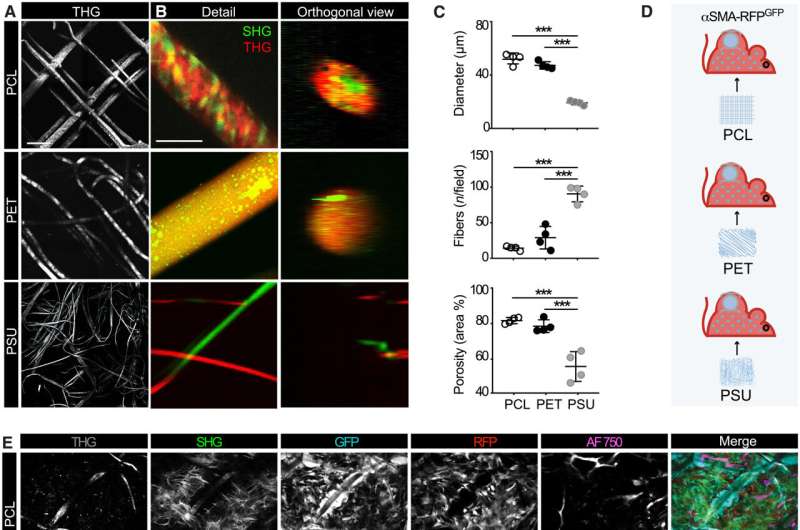
The engagement of αSMA in response to the material composition
The researchers noted how the properties of biomaterials, including their composition, charge and porosity regulated the severity of the foreign body response. The scientists modulated the material type and geometry of the implant to affect the process of recruitment and self-organization of the αSMA cells. They examined this using two different, clinically relevant polymer biomaterials. Based on nonlinear intravital multiphoton microscopy, the team observed the gradual infiltration of the fluorescently labeled smooth muscle alpha actin cells, followed by the deposition of collagen and the growth of neovessels.
Their work further sought to establish if the materials promoted the polarization of macrophages to types M1 and M2, which inhibit cell proliferation during tissue damage while promoting cell proliferation during wound healing, respectively.
Outlook
In this way, Maria Parlani and colleagues noted how fibroblasts are known effectors of fibrosis during the foreign body response, and formed a step-wise response during wound healing. Nevertheless, their process of recruitment and activation has remained relatively unknown.
Using the nonlinear intravital multiphoton microscopy technique, the scientists closed a knowledge gap to dissect the contributions of fibroblasts to promote the foreign body response, and identify the step-wise organization and activation of a biologically conserved process. The work outlines the alpha smooth muscle actin expressing fibroblasts to be a persistent element of the foreign body response. The recruitment activation and self-organization of cells around the porous implant material resulted in a biologically conserved two-part process.
More information: Maria Parlani et al, Dissecting the recruitment and self-organization of αSMA-positive fibroblasts in the foreign body response, Science Advances (2022). DOI: 10.1126/sciadv.add0014
Arturo J Vegas et al, Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates, Nature Biotechnology (2016). DOI: 10.1038/nbt.3462
Journal information: Nature Biotechnology , Science Advances
© 2023 Science X Network