Reducing molten salt's corrosive effect
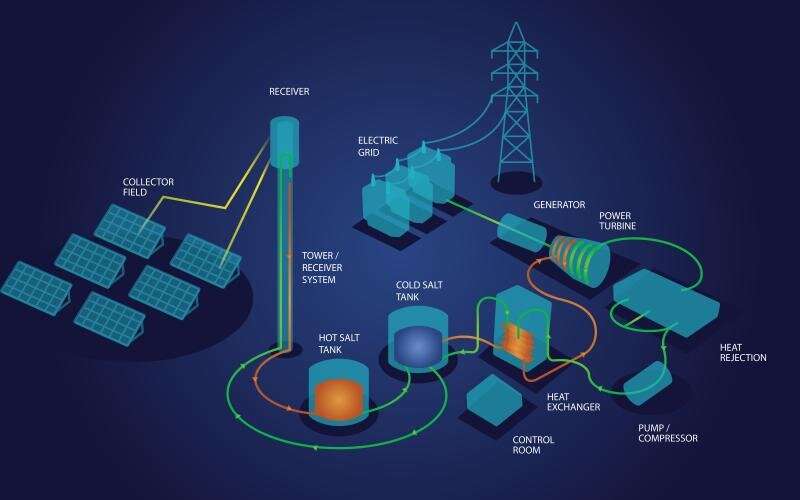
Oak Ridge National Laboratory scientists recently demonstrated a low-temperature, safe route to purifying molten chloride salts that minimizes their ability to corrode metals. This method could make the salts useful for storing energy generated from the sun's heat.
The experiment, detailed in Frontiers of Chemical Engineering, involved using thionyl chloride to remove corrosion-causing impurities from the salts. Without this purification, the salts corrode pipes and storage tanks.
The team melted a commercial carnallite—an abundant mineral being considered for solar-thermal energy storage—and put it into contact with inert gas saturated with thionyl chloride to cause a reaction. The scientists monitored reaction conditions by measuring salt temperature and by analyzing the off-gas through infrared spectroscopy.
"Using high-temperature molten salts to store energy as heat could be key in making solar energy a consistent source of electricity, replacing fossil fuels," said ORNL's Joanna McFarlane, who led the team that performed the experiment.
More information: Joanna McFarlane et al, Chloride Salt Purification by Reaction With Thionyl Chloride Vapors to Remove Oxygen, Oxygenated Compounds, and Hydroxides, Frontiers in Chemical Engineering (2022). DOI: 10.3389/fceng.2022.811513
Provided by Oak Ridge National Laboratory





















