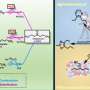
Unveiling how water and ions impact chemical reactivities at solid-aqueous interfaces
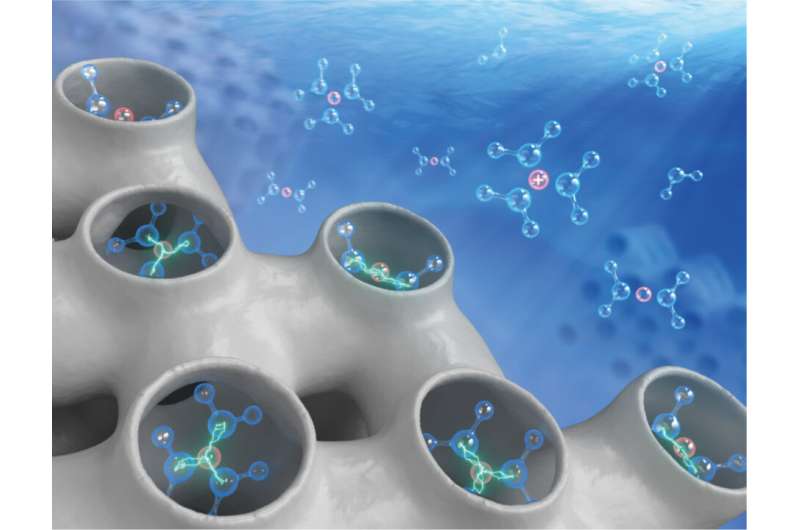
Solid-aqueous interfaces are ubiquitous and essential in a diverse range of natural and man-made systems and processes, from mineral formation, rock weathering and metal corrosion, to the intricate functioning of biological membranes and ion channels.
In all these systems and processes, water and water-borne ions play decisive roles and underpin the interfacial chemical reactivities, but oftentimes, a fundamental understanding of such roles and effects is lacking. Water is regarded as a "green" medium that is abundantly available, environmentally benign and inexpensive, so the advantage of running chemical reactions in water over organic solvents—which have dominated synthetic organic chemistry for good reasons—seems self-evident, even from a layman's point of view.
One of the key questions, however, is "can we predict with good precisions and accuracies whether water will enhance or suppress a chemical reaction over a solid catalyst?" Apparently, the answer relies on our ability to understand, in great depth, the reactivity trends at the dynamic and complex interfaces between the catalytic solid and the aqueous surroundings, which will in turn lead to the formulation and discovery of novel concepts, principles and criteria that can be leveraged upon to maximize the chemical reactivities toward the targeted product or product slates.
Despite the daunting challenges associated with this mission, the heterogeneous catalysis community has made great strides in the past decades, thanks to both the rapid development of enabling toolboxes and the intensification of interdisciplinary knowledge transfer.
So, where do we stand after decades of research on heterogeneously catalyzed reactions in the aqueous phase? Focusing on solid acid-base catalysts, which represent a subset of heterogeneous catalysts with broad applications in the chemical industry, a research team led by Prof. Hui Shi at Yangzhou University, China, compiled a comprehensive assessment of the roles of water molecules, structural moieties derived from water, and ionic species that are dissolved in it, for heterogeneous acid-base catalysis at the solid-aqueous interface.
By further restraining their attention to alcohol dehydration, aldol condensation, and sugar isomerization, all being prototypical acid-base-catalyzed reactions, these authors described an assortment of water-engendered effects, using thoughtfully picked examples, and critically discussed their chemical origins and catalytic consequences. The review was published in Chinese Journal of Catalysis.
These authors covered major advances in the atomistic and molecular-level understanding of several general modes of action, by which water affects the reaction mechanisms and structure-performance relations, drawing on the new evidence acquired for the individual set of acid-base catalysis at the solid-aqueous interface.
It is particularly important to recognize that the polar surface functionalities, silanols and cation-anion pairs for instance, and the polarity and H-bonding characteristics of the different states along the reaction path bring forth the most dramatic impact on the manifestation of water effects. By way of a comparative analysis, they concluded that for an analogous series of reactions, the roles of water may be anticipated to be generalizable from a specific subset of such chemistries. Finally, some emerging concepts that were believed to be worth exploring further were highlighted, such as "interfacial ionic strength" (or "trapore ionic strength", when the solid phase contains micropores—pores that are smaller than two nanometers or even below 1 nm).
According to Prof. Hui Shi, "although many efforts to perform reactions in water or aqueous solvents have been motivated by the inherent benignity of water as well as its availability and cost, using water as a solvent or a component of solvent mixtures should never be an ill-informed choice without a solid understanding of the chemical origins of its promotional or adverse effects."
Indeed, sometimes even a tiny drop of water is sufficient to boost the catalytic reaction dramatically, or "kill" the transformation completely. Assimilating the insights summarized in this review article will likely help researchers and practitioners synthesize a coherent picture about water-related effects and gain a better control over the practical outcome in a desired and preferred direction.
More information: Xugang Yang et al, A critical assessment of the roles of water molecules and solvated ions in acid-base-catalyzed reactions at solid-water interfaces, Chinese Journal of Catalysis (2022). DOI: 10.1016/S1872-2067(21)64032-9
Provided by Chinese Academy of Sciences