Researchers reveal activation mechanism of thyrotropin-releasing hormone receptor
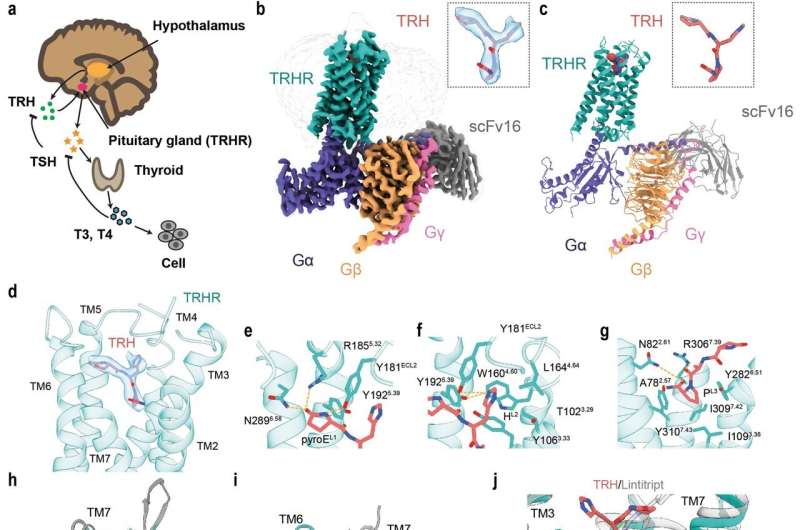
Thyroid hormone (TH) plays a multifunctional role in metabolism and development in vertebrates. The synthesis and secretion of TH is determined by the hypothalamus-pituitary-thyroid (HPT) axis. The tripeptide thyrotropin-releasing hormone (TRH, pGlu-His-Pro-NH2) is the initial hormone of HPT, synthesized in the hypothalamus and acts on TRH receptor (TRHR). Upon stimulation by TRH, TRHR prompts thyroid stimulating hormone (TSH, thyrotropin) production, which then induces the synthesis of TH.
TRHR is mainly expressed in TSH-secreting cells located at the pituitary and belongs to class A family of G protein-coupled receptors (GPCRs), which is distantly related to the other members in class A family. Activated TRHR couples Gαq and activates the phosphatidylinositol (IP3)-calcium-protein kinase C (PKC) pathway. Taltirelin, a synthetic TRH analog, has been approved for the treatment of spinocerebellar degeneration (SCD) in Japan. However, research on the other TRH derivatives were terminated. One of the important reasons is lack of structural information, especially how TRHR is recognized and activated by TRH.
In a study published in Cell Research, a team led by H. Eric Xu (Xu Huaqing) and Xu Youwei from the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences revealed the ligand binding and activation mechanism of human TRHR from a structural view.
The researchers reported the cryo-EM structure of the human TRHR bound to TRH and G protein at 3.0 angstrom resolution. Within the complex structure, the tripeptide TRH adopts a "Y-shaped" conformation with its prolinamide moiety pointing towards the helical core, which is buried in the orthosteric binding pocket composed of TM2, TM3, TM5–7, and ECL1–3. The TRHR pocket is amphipathic, with one side enriched with positively charged residues, and the other side enriched with hydrophobic residues.
Besides, the researchers analyzed the activation mechanism of TRH bound TRHR. They found that the TM6 cytoplasmic end of TM6 of the active TRHR undertakes a distinct pronounced outward displacement, as well as a lateral movement shift of TM5 and a slightly inward movement of TM7. The conformational changes could explain how TRH triggers the downstream Gαq signaling. Meanwhile, site-directed mutagenesis studies were used to verify the residues for TRH binding or activation.
The structure of the TRH-TRHR-Gq complex, combined with the finding of functional analysis, provided a basis for understanding pathogenesis caused by disease-associated mutations. Many disease-causing mutations are located in the TRH-binding pocket or at the G protein- binding interface. These results revealed detailed mechanism in TRHR activation by TRH, and first provided a starting model for investigating the interactions between TRH and its receptor TRHR and for structure-based drug design on TRHR.
More information: Youwei Xu et al, Structural insights into ligand binding and activation of the human thyrotropin-releasing hormone receptor, Cell Research (2022). DOI: 10.1038/s41422-022-00641-x
Journal information: Cell Research
Provided by Chinese Academy of Sciences