April 21, 2022 report
Nanocapsule carrying a CRISPR-Cas9 editing tool used for noninvasive brain delivery and tumor cell targeting
An international team of researchers has developed a nanocapsule that is capable of crossing the blood brain barrier (BBB) to carry the CRISPR-Cas9 editing tool to treat a brain tumor. In their paper published in the journal Science Advances, the group describes how they created their capsule and how well it worked when tested in mice with a glioblastoma.
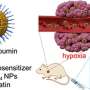
Glioblastomas are notoriously difficult to treat. The tumors appear in the brain and their growth damages tissue. Treatment options include surgical removal, direct injections of therapies meant to kill the cancer cells or inserting CRISPR viruses into the bloodstream. Each of these options has a downside, from damaged brain tissue to ineffectiveness due to difficulties with therapies crossing the BBB. In this new effort, the researchers have tried a new approach, using a nanocapsule to carry the CRISPR-Cas9 editing tool to the brain tumor where it targets a gene that is responsible for the development of new cells—a capsule that is capable of safely crossing the BBB.
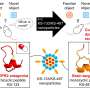
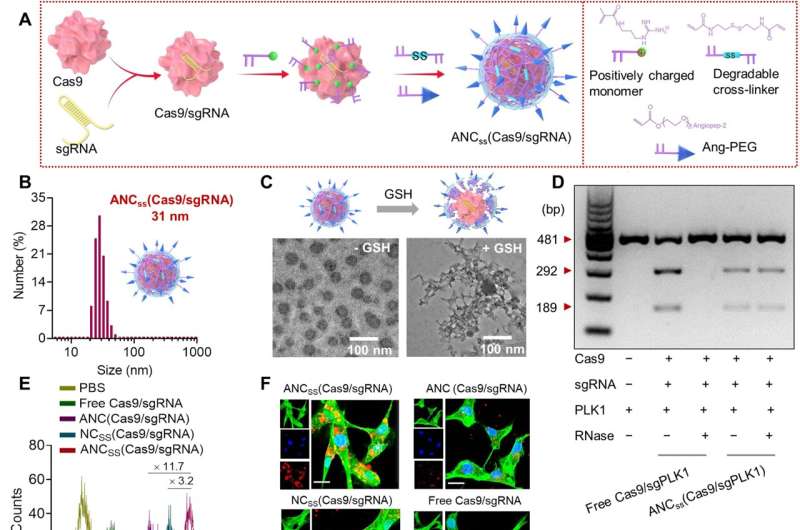
The shell of the nanocapsule was created using a disulfide, cross-linked polymer that was then dotted with an angiopep-2 peptide. The peptide was added to create a neutral surface charge so that it would not be attacked by ribonuclease. The shell was just large enough to hold a Cas9 complex but still small enough (approximately 30 nanometers in length) to allow it to pass through the BBB.
The researchers tested their nanocapsule delivery system in glioblastoma mouse models. Each was given a single tail injection—some received the newly developed nanocapsule delivery system while others received a control. The researchers found that the mice given the new therapy had a mean survival time of 68 days compared to 24 days for the control group. They also found a less than 0.5% undesired genetic mutation rate in other brain tissue.
The researchers suggest their work represents a new step toward noninvasive and nonviral approaches to treating glioblastomas, though they acknowledge that much more work needs to be done before it can be determined if the same approach will be both safe and effective in humans.
More information: Yan Zou et al, Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy, Science Advances (2022). DOI: 10.1126/sciadv.abm8011
Journal information: Science Advances
© 2022 Science X Network