Structural insights into the assembly of cilia
Cilia, the little "hairs" attached to almost all cells of the human body, play a role in various cellular functions and cause diseases called ciliopathies when they are defective. Researchers from the group of Patrick Matthias and the FMI Structural Biology platform determined the structure, at near atomic resolution, of a protein complex that plays an essential role in the assembly of cilia—and causes ciliopathies when it is mutated.
Cilia are hair-like structures that extend from the surface of almost all cell types of the human body. In addition to being "motors" that enable cell propulsion and fluid movement, cilia act as cellular antennae to sense environmental cues, for example during development, and are essential for passing on signals.
In line with their crucial functions, genetic defects in cilia give rise to more than 30 inherited human diseases termed ciliopathies. These include single organ diseases and complex syndromes that can manifest as hydrocephaly, infertility, respiratory problems, diseases of the eye, heart, kidney, and more. The identification of the components involved in cilia-specific functions and of the molecular mechanisms underlying the various ciliopathies are likely to facilitate the development of novel therapeutic strategies.
Ciliopathies are often caused by defects in cilia assembly, a process termed ciliogenesis. This complex event is under strict regulation in space and time and involves dozens of proteins. Three proteins called CPLANE proteins play a particularly important role in governing ciliogenesis, but little is known about them.
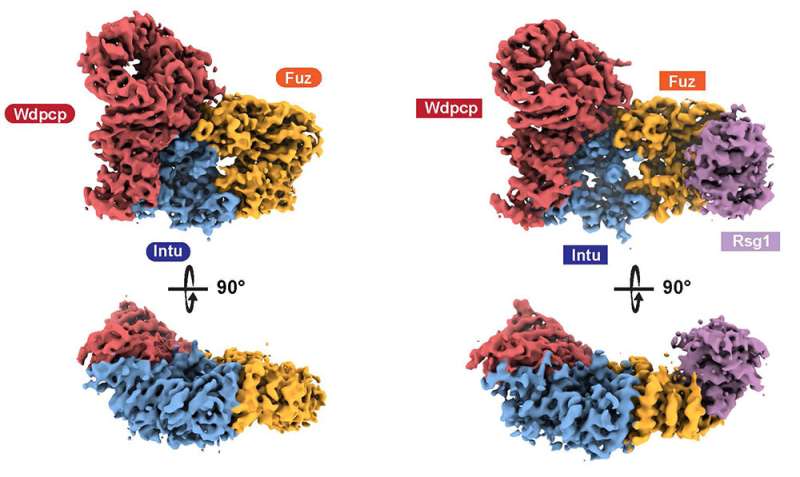
Gerasimos Langousis, postdoc in the lab of Patrick Matthias, and colleagues from the FMI Structural Biology platform used cryogenic-Electron Microscopy (cryo-EM) to study CPLANE. They showed that the three proteins assemble to form a complex they called the CPLANE complex. They determined the structure—at near-atomic resolution—of the human and the mouse complex bound to a small Rab GTPase, an enzyme that is essential for regulating cellular activity. They also studied how the complex binds to phospholipids, the key components of cell membranes (where the cilia are anchored), and what may go wrong in ciliopathies. They could demonstrate that a ciliopathy CPLANE mutant protein exhibits altered phospholipid binding.
These results illuminate how the CPLANE complex orchestrates lipid binding and Rab signaling. The study, published in Science Advances, provides critical structural and functional insights into the enigmatic process of ciliogenesis as well as new molecular rationales for ciliopathies.
More information: Gerasimos Langousis et al, Structure of the ciliogenesis-associated CPLANE complex, Science Advances (2022). DOI: 10.1126/sciadv.abn0832
Journal information: Science Advances