Haber-Bosch at the atomic scale
Industrial production of NH3 has been performed by the Haber-Bosch process for more than 100 years, in which dissociation of N2 feedstock molecules promoted by alkali atom co-catalyst is thought to be the rate limiting step. The Haber-Bosch synthesis consumes 1% of the world's total energy consumption, and accounts for 1.4% of the global CO2 emissions. Therefore, the atomic scale insights into the K atom-N2 molecule interactions on metal substrates and specifically, the alkali atom promotion chemistry, has global significance.
Researchers at the University of Pittsburgh, together with theoretical collaborators at the University of Science and Technology of China have investigated the Haber-Bosch catalysis precursor at the atomic scale.
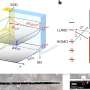
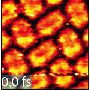
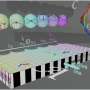
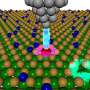
In research article to be published in Cell Reports Physical Science on April 21, 2022, the researchers, led by Hrvoje Petek at the University of Pittsburgh, were able to directly observe at the atomic scale by scanning tunneling microscopy the N2 adsorption, their collective interactions, and tunneling electron-induced N2 desorption processes that are related to the alkali promotion of NH3 synthesis.
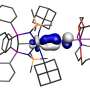
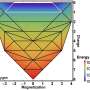
The dominant pairwise interaction between the K and N2 is an electrostatic, two-center, Coulomb attraction, where charge transfer from K to N2 weakens the N2 molecule bond towards its dissociation in the Haber-Bosch synthesis. The K-N2 interactions interpreted through density functional theory are in agreement with the experimental observations.
The studies reveal the primary interactions, as well as the onset of correlated complexity that defines the alkali atom promotion of catalytic chemistry.
More information: Chao Zhang et al, Imaging a Haber-Bosch catalysis precursor at the atomic scale, Cell Reports Physical Science (2022). DOI: 10.1016/j.xcrp.2022.100865
Journal information: Cell Reports Physical Science
Provided by University of Pittsburgh