Is it possible to develop antiaromaticity by nonbenzenoid aromatic compounds?
Circularly conjugated compounds with s are known as aromatic compounds. Since they are generally stable, they are widely used in our daily life, from plastics to pharmaceuticals, dyes, and organic electronics materials. On the other hand, antiaromatic compounds with 4n pi-electrons are not stable and thus are known to decompose or change their structures, which leads to losing their "antiaromatic" properties. From recent studies, it has been proven that antiaromatic compounds exhibit absorption in the near-infrared region, high charge transport properties, and redox properties, but only a limited number of examples is reported.
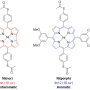
Our research group has studied the synthesis and characterization of hexapyrrolohexaazacoronene (HPHAC), a nitrogen-containing polycyclic aromatic compound, using pyrroles. HPHACs, which are composed of electron-rich pyrroles, are easily oxidized, and their two-electron oxidized form (dication) exhibits aromaticity based on macrocyclic conjugation. In general, their properties as "aromatic" or "antiaromatic" compounds can be converted by the number of pi-electrons, and this can be conducted by the redox process (electron donation and acceptance). Our research group has also reported that homoHPHAC, a pi-extended analog of HPHAC, is stable and exhibits strong antiaromaticity.
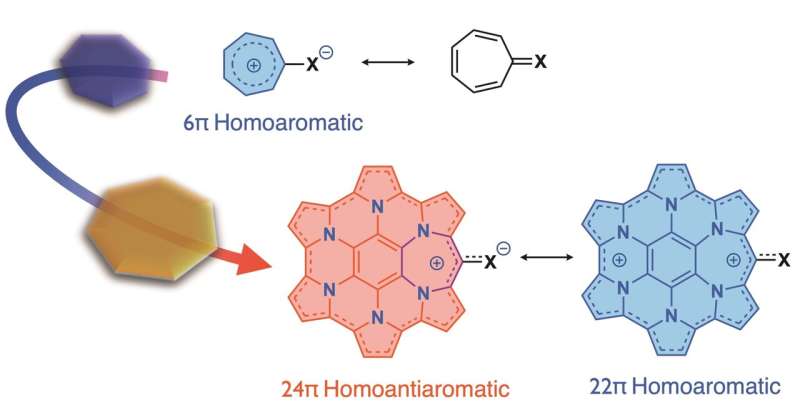
In this study, tropone (tropothione)-embedded homoHPHACs were synthesized and their structures were characterized, and their redox and aromatic properties were elucidated. Tropone (tropothione) is known to form a nonbenzenoid aromatic compound, tropylium cation (6pi-electron aromatic ring), when the carbonyl (thiocarbonyl) group is polarized to form a partially positive charge on the carbon atom and a partially negative charge on the oxygen (sulfur) atom. A detailed study of the properties of the compounds revealed that, although they did not exhibit antiaromaticity through interaction with various solvents or Lewis acids, a clear antiaromaticity was observed upon methylation of the thiocarbonyl group. This indicates that the antiaromaticity of the homoHPHAC moiety was induced by the formation of the tropylium cation upon methylation.
Various approaches to the use of organic compounds as electronics materials are being investigated from the viewpoints of reducing environmental impact and diversity of functional control. If it becomes possible to switch the physical properties between "aromatic" and "antiaromatic" without transferring electrons, it will pave the way for the development of new applications.
The research was published in Chemical Communications.
More information: Masayoshi Takase et al, Tropo(thio)ne-embedded homoHPHACs: does the tropylium cation induce global antiaromaticity in expanded hexapyrrolohexaazacoronene?, Chemical Communications (2022). DOI: 10.1039/d1cc07152a
Journal information: Chemical Communications
Provided by Ehime University