New catalysts steer hydrogen fuel cells into mainstream
Cornell chemists have discovered a class of nonprecious metal derivatives that can catalyze fuel cell reactions about as well as platinum, at a fraction of the cost.
This finding brings closer a future where hydrogen fuel cells efficiently power cars, generators and even spacecraft with minimal greenhouse gas emissions.
"These less expensive metals will enable wider deployment of hydrogen fuel cells," said Héctor D. Abruña, the Émile M. Chamot Professor in the Department of Chemistry and Chemical Biology in the College of Arts and Sciences. "They will push us away from fossil fuels and toward renewable energy sources."
Abruña, with co-authors Francis DiSalvo, the John A. Newman emeritus professor of chemistry; doctoral student Rui Zeng; Yao Yang, Ph.D. '21; Xinran Feng, Ph.D. '21, and Huiqi Li, a visiting graduate student from Xiamen University; and Lauryn M. Gibb '22, published the findings in the journal Science Advances.
As long as combustion engines rule the streets and fill the skies with smog, it is hard to imagine a sustainable future for transportation. Hydrogen fuel cells, which convert hydrogen directly into electricity with only water and a small amount of heat as byproducts, are promising renewable alternatives.
A critical part of the fuel cell is the oxygen reduction reaction (ORR), an infamously sluggish process—Abruña often calls it "God's collective punishment to electrochemistry"—that is traditionally sped up by platinum and other precious metals. A model catalyst, platinum conducts electricity, catalyzes the most temperamental reactions with aplomb, and is hardy enough to survive the harsh, acidic environment of a fuel cell. But it can be prohibitively expensive.
Lately, however, more forgiving alkaline fuel cells have gained prominence, raising the possibility that less expensive metals, once ruled out for their vulnerability to acidic environments, might replace platinum in these gentler, next-generation fuel cells. Abruña and his team set out to engineer an inexpensive material, fit for an alkaline fuel cell, that would conduct electricity and catalyze the ORR reaction just as efficiently as platinum.
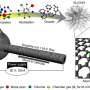
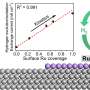
Transition metal nitrides (TMNs) were an obvious choice, and DiSalvo is a world expert on these materials, Abruña said. A class of compounds derived from cobalt, manganese, iron and other transition metals, TMNs conduct electricity and, when exposed to air, tend to form a thin oxygen-based outer shell that provides a perfect surface for catalyzing chemical reactions. After synthesizing a family of TMNs with conductive nitride cores and reactive oxide shells, the team tested each candidate catalyst in a model hydrogen fuel cell.
Manganese- and iron-based candidates made strong showings. But the cobalt nitride catalyst was "the clear winner," Abruña said, with near identical efficiency to platinum while costing 475 times less as of Feb. 2.
Those savings may help finally brings hydrogen fuel cells out of the laboratory and into the mainstream. If affordable, fuel cells could replace combustion engines and car batteries with a sustainable alternative that, fed a steady diet of hydrogen, never needs to recharge and wastes as little as 10% of the energy that goes into making it run. By comparison, a typical car engine wastes about 75% of its energy.
"Hydrogen fuel cells are enormously powerful, enabling you to run at an efficiency that simply does not exist for more traditional engines," Abruña said. "People recognize that fuel cells are the way to go. The trick is designing stable and affordable catalysts that make it all possible."
More information: Rui Zeng et al, Nonprecious transition metal nitrides as efficient oxygen reduction electrocatalysts for alkaline fuel cells, Science Advances (2022). DOI: 10.1126/sciadv.abj1584
Journal information: Science Advances
Provided by Cornell University