Method of molecular-level control can double the efficiency of widely used industrial catalysts
The science of catalysis—the acceleration of a chemical reaction—is perhaps not the most recognizable branch of study, but it is absolutely embedded into the fabric of modern society.
The development and production of fuels, chemicals, pharmaceuticals and other goods depend on catalysis. Catalysis plays a critical role in energy generation and the mitigation of humanity's impact on the environment, and is involved in the manufacturing of some 25 percent of all industrial products in the U.S. From a consumer's perspective, if a thing is made, worn, lived in, played with, driven upon, or otherwise used by people, catalysis likely plays a fundamental role in its origin story.
Research in the field of catalysis enables new and improved products and more efficient ways of doing and manufacturing, well, just about everything. But with such deep entanglement in the world around us, advancement in industrial catalysis can be costly in a macroeconomic sense—wholesale changes that require a "rip and replace" strategy do not sit well with firms and supply chains that power and provision our modern economy.
In a paper published online today (20 January 2022) in Nature Catalysis, researchers from Lehigh University, in collaboration with colleagues from the East China University of Science and Technology (ECUST), propose a novel method of significantly enhancing the catalytic efficiency of materials already in broad commercial usage, a process they have termed "induced activation."
The article, "Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol," The research team, supported by the National Natural Science Foundation of China and the U.S. Department of Energy's Office of Science, includes Israel E. Wachs, the G. Whitney Snyder Professor of Chemical and Biomolecular Engineering at Lehigh University, Ph.D. student Tiancheng Pu of Lehigh's Operando Molecular Spectroscopy and Catalysis Research Lab, and Minghui Zhu, a 2016 Lehigh Ph.D. who now serves as a professor of chemical engineering at ECUST. Other collaborating ECUST researchers include Didi Li, Fang Xu, Xuan Tang, Sheng Dai, Xianglin Liu, Pengfei Tian, Fuzhen Xuan, and Zhi Xu.
Induced activation: A game-changer in the control of catalytic surface
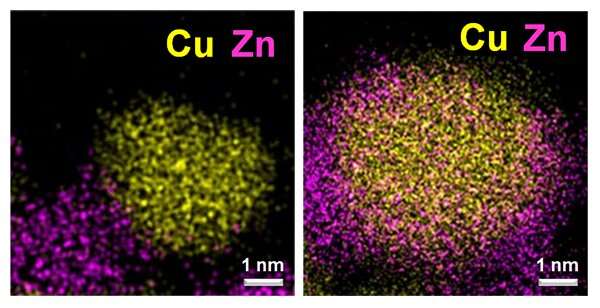
"The surface structure of heterogeneous catalysts is closely associated with their catalytic performance," explains Wachs. "Current efforts for structural modification mainly focus on improving catalyst synthesis. Induced activation, on the other hand, takes a different approach—manipulating the catalyst surface by controlling the composition of reducing agents at the catalyst activation stage where the catalyst is transformed to its optimum state."
The team says that the use of the "tried and true" industrial catalytic material copper/zinc oxide/aluminum oxide (Cu/ZnO/AlO3) enables firms to take advantage of the breakthrough without the need for a costly retooling.
"This development effectively doubles the catalytic efficiency of these materials, enhancing their productivity and extending the life of the catalyst," Wachs says. "And importantly, induced activation can provide significant benefit to industry without shutting down a chemical plant—or the building of a new and costly one."
More information: Didi Li et al, Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol, Nature Catalysis (2022). DOI: 10.1038/s41929-021-00729-4
Journal information: Nature Catalysis
Provided by Lehigh University