A new method for engineering enzyme stereoselectivity and substrate acceptance
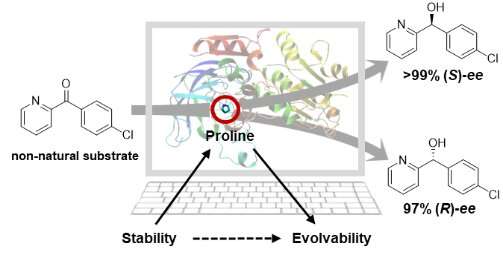
Researchers led by Prof. Sun Zhoutong at the Tianjin Institute of Industrial Biology of the Chinese Academy of Sciences have developed Proline Induced Loop Engineering Test (PiLoT) method that helps to discover additional hotspots beyond the conventional ones around the active site.
The designed mutagenesis is most likely to generate variants capable of inducing conformational dynamics, according to the researchers.
Natural enzymes have progressively evolved to fit particular catalytic reactions during evolutionary trajectories with novel functions. In some cases, the underlying evolvability was unlocked via altering stability by specific amino acid substitutions. However, identifying particular mutational positions in a protein to tailor its stability on a tolerable scale that triggers conformational plasticity, and thereupon novel functions, is a fundamental challenge.
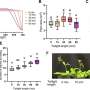
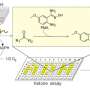
In this study, the thermophilic alcohol dehydrogenase TbSADH was employed as the biocatalyst in the stereoselective transformation of the difficult to reduce diaryl ketone, which is of pharmaceutical significance. The proline residue (P84) situated at the interface of a β-strand and loop region was mutated, the resultant mutants leading to unusually high (S)- and (R)-selectivity, respectively, amounting to 99% ee and high conversion.
Combined computational analysis revealed that these mutants gained more flexibility near the active site and thereby provided extra space for the acceptance of a non-natural bulky-bulky substrate.
Results show that PiLoT constitutes a new tool in the rational mutagenesis of enantiocomplementary alcohol dehydrogenase (ADH) enzyme mutants. It allows the tailoring of sufficient ADH stability for enabling the necessary evolvability.
This research has been published online in Angewandte Chemie International Edition.
More information: Ge Qu et al, Unlocking the Stereoselectivity and Substrate Acceptance of Enzymes: Proline Induced Loop Engineering Test, Angewandte Chemie International Edition (2021). DOI: 10.1002/anie.202110793
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences