Making plastic durable and degradable
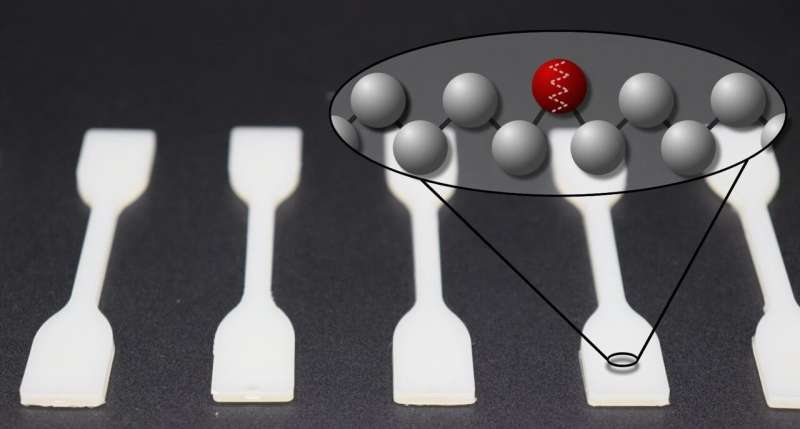
Polyethylene is the most abundantly manufactured plastic in the world. Due to properties like durability, it has many diverse, and even long-term uses. Professor Stefan Mecking's team in the Department of Chemistry at the University of Konstanz has now incorporated polar groups in the material's molecular chains in order to expand its properties and simultaneously reduce the problematical persistence of plastic in the environment. The desired favorable properties of polyethylene remain unchanged afterwards. Results of this laboratory study have been published in Science on 29 October 2021.
Polyethylene is a non-polar, hydrophobic material that—like wax—is water-repellent. To expand its material properties, by for instance improving adhesion to metal surfaces, ways have long been sought to incorporate a small amount of polar groups in the material during polyethylene synthesis. To date, this has been most elusive as conventional catalysts used in the process were destroyed by polar reagents.
Small amounts of carbon monoxide and a suitable catalyst
Doctoral researchers Maximilian Baur, Tobias O. Morgen and Lukas Odenwald, together with post-doctoral Humboldt research fellow Fei Lin in the Chemical Materials Science research team, have now succeeded in incorporating keto groups in the molecular chains that plastics are comprised of. This is achieved by using a catalyst which—due to its position in the periodic table of elements—is compatible with the carbon monoxide utilized as a reagent for producing keto groups.
A key factor in this process is creating just a limited amount of keto groups in order to maintain typical and favorable mechanic properties of polyethylene like durability. "Science and technology have long sought after a method to incorporate such groups in polyethylene chains. Our current achievement now opens up new perspectives," Stefan Mecking concludes.
New plastic with better degradability
Another special feature is that the limited amount of keto groups can also improve the new plastic's degradability. On a laboratory scale it was demonstrated that, when exposed to simulated sunlight, the new plastic displays a slow chain degradation which does not occur in conventional polyethylenes. "This material provides a new approach for developing non-persistent polyethylene. Further studies are definitely required, in order to also understand long-term performance," Stefan Mecking cautiously remarks.
Meanwhile, in their laboratory studies the team demonstrated that the new material has the same favorable properties as conventional polyethylene, as far as mechanics and processability are concerned.
More information: Maximilian Baur et al, Polyethylene Materials with In-Chain Ketones from Non-Alternating Catalytic Copolymerization, Science (2021). DOI: 10.1126/science.abi8183
Journal information: Science
Provided by University of Konstanz