Using LAMP to reveal the mysteries of lysosomes
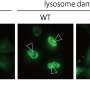
A cell is composed of numerous organelles, each with a unique role that helps contribute to its overall functionality. The lysosome is an organelle that contains digestive enzymes and functions as a molecular garbage disposal and recycling center. Since the role of lysosome is crucial to maintaining the cellular homeostasis, the lysosomal dysfunction causes neurodegenerative and metabolic diseases, cancer, and lysosomal storage disorders.
In a new article published in Autophagy, researchers at Tokyo Medical and Dental University (TMDU) performed a novel type of structural analysis to demonstrate how a certain molecular interaction is crucial for one lysosomal membrane protein to perform effectively.
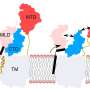
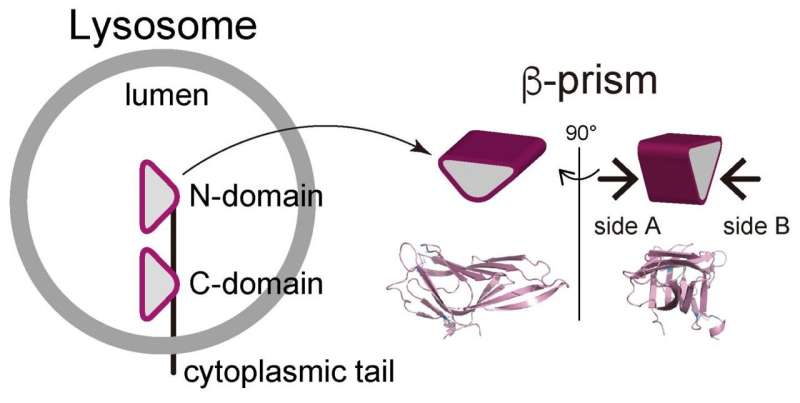
LAMP1 (lysosomal-associated membrane protein 1) and LAMP2 are the most abundant protein components of lysosome membranes. Both LAMP1 and LAMP2 are composed of a large lumenal domain, a transmembrane domain, and a short C-terminal cytoplasmic tail. The lumenal domains of LAMPs are composed of two domains (N-domain and C-domain, which are membrane-distal and -proximal, respectively). Each domain has the unique β-prims fold structure (a triangular prism). On the other hand, genetic experiments have shown that mice embryos without both LAMP-1 and 2 die a little more than two weeks after fertilization. Mice without LAMP-1 are born and can thrive, while those without LAMP-2 often die a few weeks after birth, suggesting that LAMP-2 is more important for lysosome activity. The research of the TMDU group further investigates this protein's biological role.
"Mice lacking LAMP-2 also display issues with autophagy progression," says one of the lead authors of the study Kazue Terasawa. "Expanding our knowledge of how LAMP-2 interacts and operates at the molecular level will ultimately help us understand autophagy better."
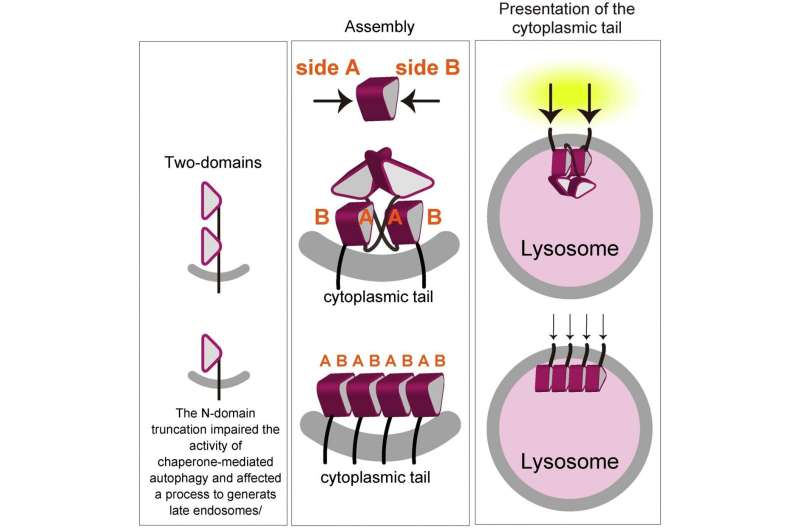
The researchers demonstrated LAMP2 molecules assemble by facing each other with one side the β-prism (defined as side A) of the C-domain. The N-domain truncation permitted the nonspecific involvement of both sides of the β-prims (side A and side B). In combination with some biochemical studies, "we believe that the homophilic interaction we demonstrated is crucial for function of LAMP-2, via ensuring a proper arrangement of the cytoplasmic tails, which is crucial for the function of LAMP2, on the lysosome membrane," says Miki Hara-Yokoyama, senior author.
More information: Kazue Terasawa et al, Direct homophilic interaction of LAMP2A with the two-domain architecture revealed by site-directed photo-crosslinks and steric hindrances in mammalian cells, Autophagy (2021). DOI: 10.1080/15548627.2021.1911017
Provided by Tokyo Medical and Dental University