Scientists shed light on the mechanism of photoactivation of the orange carotenoid protein
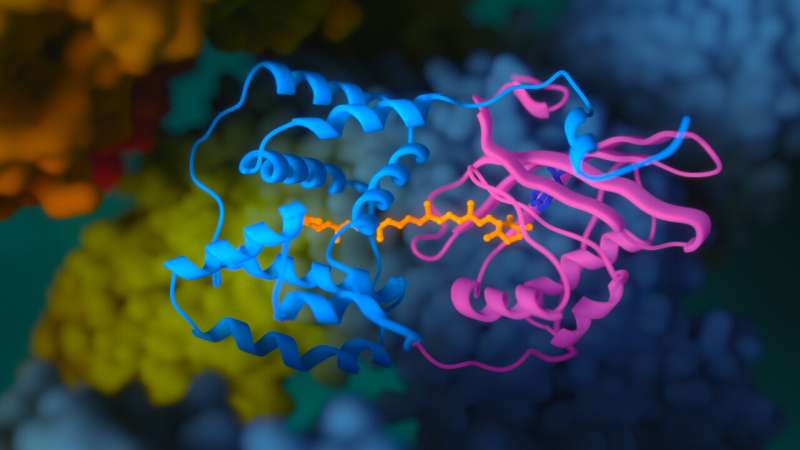
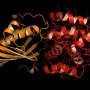
Exposure to light is compulsory for photosynthetic organisms for the conversion of inorganic compounds into organic ones. However, if there is too much solar energy, the photosystems and other cell components could be damaged. Thanks to special protective proteins, the overexcitation is converted into heat—in the process called non-photochemical quenching. The object of the published study, OCP, was one of such defenders. It was first isolated in 1981 from representatives of the ancient group of photosynthetic bacteria, yanobacteria. OCP comprises two domains, forming a cavity in which a carotenoid pigment is embedded.
"When light is absorbed by the carotenoid molecule, OCP can change from an inactive orange to an active red form. This process is multi-stage and follows a complex hierarchy of events. We showed the asynchrony of these changes in previous work, but the mechanism of the very first stage of OCP activation, associated with the breakage of hydrogen bonds between the carotenoid and the protein, remained unsolved," says Dr. Eugene Maksimov, Senior Researcher of the Federal Research Centre of Biotechnology of RAS.
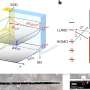
Scientists conducted a comprehensive study using methods of structural biology, biochemistry, spectroscopy, and quantum chemistry. Researchers from the Federal Research Centre for Biotechnology, Russian Academy of Sciences, have created a "super orange" version of OCP with unique spectral and structural properties and determined its crystal structure with the highest spatial resolution among all OCP-related proteins.
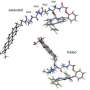
The analysis of the obtained data revealed that a charge separation reaction could occur along the hydrogen bond between the carotenoid molecule and one of the amino acid residues of the protein as a result of the absorption of a photon in OCP. In darkness, this hydrogen bond stabilizes the orange OCP state, but upon illumination, it breaks quickly due to the redistribution of the electron density in the carotenoid molecule. As a result, the protein becomes a dipole, which leads to a change in its entire structure. This photochemical reaction has been described for carotenoids for the first time.
"Our discovery will allow controlling the process of OCP activation and its spectral properties. Consequently, this can lead to the creation of new light-controlled systems and 'smart' biocompatible materials based on photoactive proteins for optogenetics and functional imaging," says Dr. Nikolai Sluchanko, Leading Researcher of the Federal Research Center of Biotechnology of RAS.
More information: Igor A. Yaroshevich et al, Role of hydrogen bond alternation and charge transfer states in photoactivation of the Orange Carotenoid Protein, Communications Biology (2021). DOI: 10.1038/s42003-021-02022-3
Journal information: Communications Biology
Provided by Federal Research Centre "Fundamentals of Biotechnology"