Multidisciplinary cooperation leads to catalysts that are up to 50 times more effective
A team of chemists and physicists at Utrecht University has succeeded in designing a new type of catalyst. By combining two metals with atomic precision they created a highly effective catalytic material. The team, led by Prof. Petra de Jongh (Chemistry) and Prof. Alfons van Blaaderen (Physics), are publishing their findings in Nature Materials today.
Nanoparticles
Catalysts strongly impact our society. Approximately 90% of all industrial chemical processes make use of a catalyst to accelerate chemical conversions. These catalysts typically contain tiny metal particles, called nanoparticles, which are about 10,000 times smaller than the width of a human hair. The structure and composition of these nanoparticles determine how good the catalyst is. Even small changes in these nanoparticles can lead to large differences in performance, therefore posing significant economic and environmental implications for our society.
Bimetallic catalysts: when two is better than one
"An important development in improving the performance of catalytic materials is to move from conventional catalysts made of a single metal to bimetallic catalysts in which two different metals are combined," explains Petra de Jongh. These bimetallic catalysts work better, but also give rise to new challenges. "The challenge is that with conventional techniques you have little control over the structure of the nanoparticles, resulting in particles with varying amounts of both metals, and different shapes and sizes, severely hampering the efficiency of these bimetallic catalysts." Designing bimetallic catalysts with atomic precision was a key goal for Jessi van der Hoeven, a Ph.D. candidate who performed her research in two groups at the Debye Institute for Nanomaterials, jointly supervised by De Jongh (Chemistry) and Van Blaaderen (Physics).
Arranging the atoms in a core-shell design
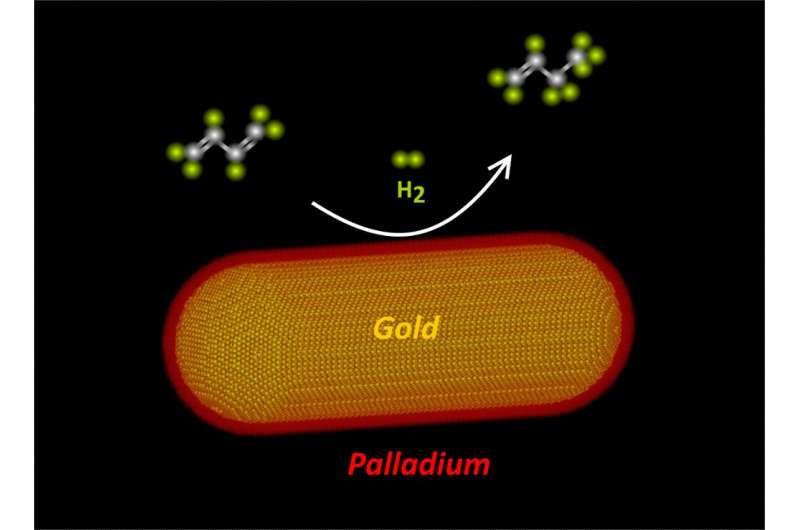
Van der Hoeven found a way to combine two metals, gold and palladium, in a core-shell structured nanoparticle while controlling the number of layers of palladium atoms. These new catalysts were tested in the selective hydrogenation of butadiene, a crucial process in purifying feedstock for making plastics. De Jongh notes, "We were very excited to see that with this core-shell design we made catalysts that perform up to 50 times better than those consisting of only gold or only palladium, or a random mixture of the two."
Van der Hoeven adds, "To our surprise we also observed that not only the type of atoms at the surface of the nanoparticle impacts the performance, but that the nature of the atoms in the layers below the surface matter as well." With the help of theorist from Karlsruhe Institute of Technology (Germany) and spectroscopists from the Sorbonne University in Paris (France), co-authors of the publication, they investigated this effect in detail.
Plenty of room at the bottom
Although the current gold-palladium core-shell catalysts exceeded their expectations, the authors are convinced that there are still many opportunities for improvement. "We are just at the beginning," comments van Blaaderen. "Now that we know how to arrange the atoms in the nanoparticles, the variety of structures and metal combinations that we can explore is enormous." Their dream for the future is to keep building catalyst materials from the bottom up, inspired by the physicist Richard Feynman, who already predicted that "there is plenty of room at the bottom" for humanity to build materials up atom by atom. Or in this case, layer by layer.
More information: Jessi E. S. van der Hoeven et al. Unlocking synergy in bimetallic catalysts by core–shell design, Nature Materials (2021). DOI: 10.1038/s41563-021-00996-3
Journal information: Nature Materials
Provided by Utrecht University Faculty of Science