Researchers gain insight into how solvent molecules impact light-driven reactions
Light-absorbing molecules can transform photons into electricity or fuels by shuttling electrons from one atom to another. In many cases the molecules are surrounded by a solvent—water, in the case of photosynthesis—and studies have shown that the solvent plays an important role in electron transfer. But measuring the motions of solvent molecules to find out how they influence the process has been difficult.
In a new study, researchers have captured the rapid motions of solvent molecules that impact light-driven electron transfer in a molecular complex for the first time—information that could help researchers learn how to control energy flow in molecules, potentially leading to more efficient clean energy sources.
"It's a long-standing challenge in chemistry to understand, at a microscopic level, the crucial role solvents play in chemical reactions," says Elisa Biasin, a research associate at the Stanford PULSE Institute at the Department of Energy's SLAC National Accelerator Laboratory. "Until recently we didn't have tools that were directly sensitive to atomic motion at very fast time scales to investigate this."
A research team led by Munira Khalil, a chemistry professor at the University of Washington, with collaborators at SLAC and the DOE's Pacific Northwest National Laboratory (PNNL) overcame this obstacle using a combination of X-ray techniques and simulations. They published their results in Nature Chemistry.
Synchronized motions
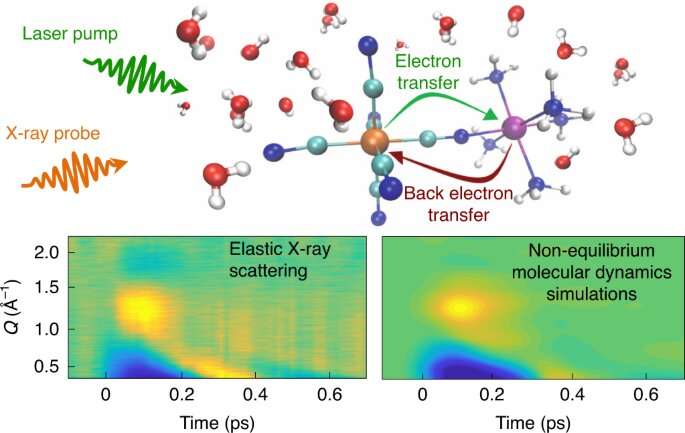
The team focused on a molecular complex containing two metal atoms that can exchange an electron between them. This system serves as a platform to study electron transfer reactions. First they dissolved the complex in water, where it formed strong hydrogen bonds with surrounding water molecules. They kicked off the electron transfer process between the metal atoms using an optical laser pulse. Then they scattered X-ray pulses from SLAC's Linac Coherent Light Source (LCLS) off the sample to monitor the motion of the atoms in the complex and the surrounding solvent molecules during the electron transfer.
The ultrashort X-ray pulses, just millionths of a billionth of a second long, captured the synchronized motions of the water molecules that were bonded to the complex. As an electron transferred from one metal atom to the other, the hydrogen bonds weakened and the solvent molecules moved away from the complex. When the electron returned to the first metal atom, the solvent molecules oscillated back to their original position.
"This is the first time that we've been able to experimentally capture a specific motion of a solvent that's in this sort of lockstep with what's happening inside the molecular complex," Khalil says.
Capturing the dance
The team was able to analyze and interpret the experimental results using molecular simulations. Physicist Niri Govind and computational chemist Amity Andersen from PNNL contributed to these simulations with NWChem, an open-source PNNL-developed computational chemistry software package.
Govind says, "The combination of experiment and molecular simulation was crucial to understanding the coupled dance that occurs during ultrafast electron transfer between the metal atoms and the surrounding water molecules."
To follow up, the researchers hope to conduct experiments with other solvents to see how they affect electron transfer.
"The goal," Biasin says, "is to learn enough at the atomic scale that we can make predictions and learn how to exert some level of control on electron transfers and other important chemical reactions."
More information: Elisa Biasin et al. Direct observation of coherent femtosecond solvent reorganization coupled to intramolecular electron transfer, Nature Chemistry (2021). DOI: 10.1038/s41557-020-00629-3
Journal information: Nature Chemistry
Provided by SLAC National Accelerator Laboratory