The 'protein from hell' tamed in seven years
After seven years of intense research, a research group from Aarhus University has succeeded—through an interdisciplinary collaboration—in understanding why a very extended structure is important for an essential protein from the human immune system. The new results offer new opportunities for adjusting the activity of the immune system both up and down. Stimulation is interesting in relation to cancer treatment, while inhibition of the immune system is used in treatment of autoimmune diseases.
In our bloodstream and tissues, the complement system acts as one of the very first defense mechanisms against pathogenic organisms. When these are detected by the complement system, a chain reaction starts, which ends with the pathogen being eliminated and other parts of the immune system being stimulated. The protein properdin is crucial for the efficiency of the complement system. Fortunately, we almost all have sufficient properdin, as otherwise, we are at risk of dying as a child from infectious diseases.
In 2013, Professor Gregers Rom Andersen from the Department of Molecular Biology and Genetics at Aarhus University was frustrated to know many details about other proteins in the complement system, but properdin seemed to be too difficult to work with. It was known that the protein had a very extended structure, which would make it almost impossible to determine the three-dimensional structure of properdin. To make matters worse, properdin is present in three different forms within the body, so-called oligomers, which contain two, three or four copies of the protein.
"But fortunately, Dennis Vestergaard Pedersen walked in the door in the autumn of 2013 to start as a Ph.D. student. Actually, he only had to work with properdin as a side project as it was too risky. But, one day we sketched on the back of an envelope how one might be able to cut properdin into smaller pieces. It worked surprisingly well," says Gregers Rom Andersen.
In this way Pedersen determined the crystal structure of the individual pieces of a properdin molecule after five years. By then, Dennis had long since finished his Ph.D. and did contract research at Aarhus University. But one thing bothered Dennis.
"Despite five years of work, I still did not know what the properdin oligomers we have in our body look like and what role their unusual and extended structure plays for the function of properdin," says Pedersen.
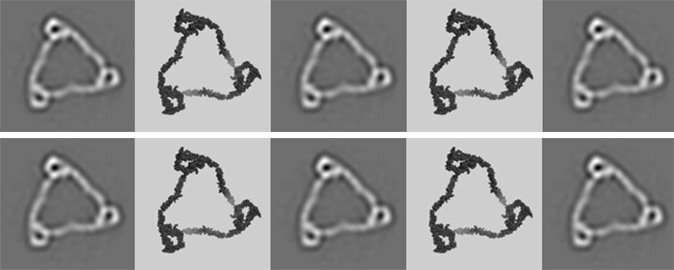
"I started for fun studying one of the three properdin oligomers using electron microscopy. To my great surprise, I discovered that this properdin oligomer is a rigid molecule, and not flexible as I had expected. This was a big surprise, and really whetted my appetite," Pedersen continues.
"In collaboration with colleagues at the University of Copenhagen, I therefore started to study the various properdin oligomers in detail. We combined electron microscopy and a technique called small angle scattering, and we were thus able to prove that all the different properdin oligomers are rigid, spatially well-defined molecules."
The special extended structure of properdin is important for the function of the protein in the immune system
At the same time, Pedersen—along with Master thesis student Sofia MM Mazarakis—conducted laboratory experiments comparing the ability of artificial and naturally occurring properdin oligomers to drive the activation of the complement system. By combining these data with structural data, the researchers were able to show that the special extended structure of properdin actually is important for the function of the protein in the immune system.
Gregers Rom Andersen elaborates: "Dennis' long and persistent work with properdin has given us a whole new understanding of 'the molecule from hell' and especially why it looks the way it does. It has given us new opportunities in terms of being able to control the activity of the complement system both up and down. Stimulation is interesting in connection with cancer, while shutdown of the complement is already used to treat autoimmune diseases."
Throughout his long work with properdin, Pedersen has collaborated with a large pharmaceutical company and will now try to develop medicine himself. Together with three other researchers from Aarhus University, he has developed a technique that takes advantage of our body's own complement system to kill cancer cells. The technique has just been patented by Aarhus University, and the researchers are now further developing the technique with support from the Innovation Fund Denmark and the Novo Nordisk Foundation.
More information: Dennis V Pedersen et al, Properdin oligomers adopt rigid extended conformations supporting function, eLife (2021). DOI: 10.7554/eLife.63356
Journal information: eLife
Provided by Aarhus University


















