October 8, 2020 feature
Giant electrochemical actuation in a nanoporous silicon-polypyrrole hybrid material
The absence of piezoelectricity in silicon can lead to direct electromechanical applications of the mainstream semiconductor material. The integrated electrical control of silicon mechanics can open new perspectives for on-chip actuators. In a new report, Manuel Brinker and a research team in physics, materials, microscopy and hybrid nanostructures in Germany, combined wafer-scale nanoporosity in single-crystalline silicon to synthesize a composite demonstrating macroscopic electrostrain in aqueous electrolytes. The voltage-strain coupling was three-orders of magnitude larger than the best performing ceramics. Brinker et al. traced the electro-actuation to the concerted action of a 100 billion nanopores-per-square-centimeter cross-section and obtained exceptionally small operation voltages (0.4 to 0.9 volts) alongside sustainable and biocompatible base materials for biohybrid materials with promising bioactuator applications. The work is now published on Science Advances.
Developing polymers with embedded electrochemical actuation
Electrochemical changes that occur during the oxidation of the conductive polymer polypyrrole (PPy) can increase or decrease the number of delocalized changes in the polymer backbone. When immersed in an electrolyte, the material is accompanied with reversible counter-ion uptake or expulsion with macroscopic contraction as well as swelling under electrical potential control to make PPy one of the most common materials to develop artificial muscle materials. In this work, Brinker et al. combined the actuator polymer with a three-dimensional (3-D) scaffold structure of nanoporous silicon to design a material for embedded electrochemical actuation. The new construct contained a few light and abundant elemental constituents including hydrogen (H), carbon (C), nitrogen (N), oxygen (O), silicon (Si) and chlorine (Cl).
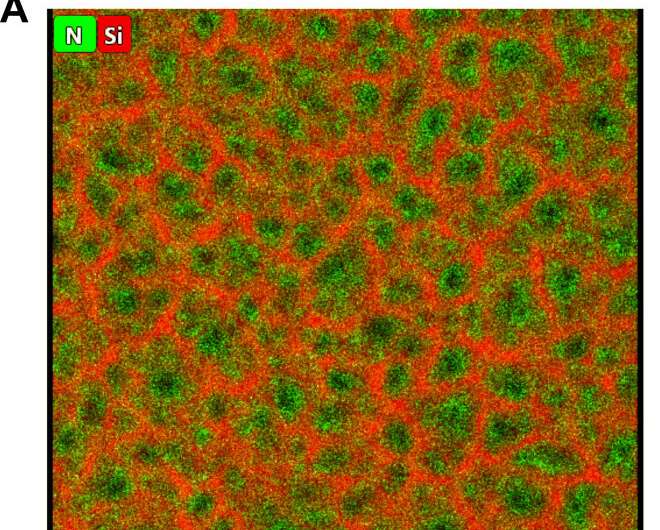
During the experiment, the team prepared the porous silicon (pSi) membrane using an electrochemical etching process of doped silicon in hydrofluoric acid. The resulting pores were straight and perpendicular on the silicon surface. Using scanning electron microscopy profiles, Brinker et al. observed a homogenous sample thickness. They then filled the porous silicon (pSi) membrane with polypyrrole (PPy) through electropolymerization of pyrrole monomers. Polymer nucleation and partial oxidation of pSi increased the open circuit potential leading to a constant deposition of PPy inside the pores. The highly asymmetrical pores formed a chain-like polymer growth inhibiting the branching of the polymer and leading to lower electrical resistance. The team observed the resulting composite using transmission electron micrographs (TEM) with energy-dispersive X-ray (EDX) spectroscopy signals to indicate homogeneous PPy filling of the random pSi honeycomb structure.
Characterizing the hybrid material
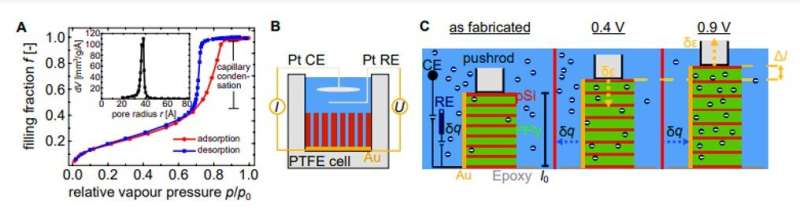
To characterize the function of the resulting hybrid material, Brinker et al. performed dilatometry measurements; a thermo-analytical method to measure the shrinking or expansion of materials, in an in situ electrochemical setup. They immersed the sample in perchloric acid and positioned it so that the pores pointed in a horizontal direction. The team then positioned the quartz probe of the dilatometer on top of the sample to measure its length after which they set up the sample in contact with perchloric acid to conduct electrochemical actuation experiments. Brinker et al. measured the electrochemical characteristics of the hybrid system before and during dilatometry measurements by recording cyclic voltammograms (CVs) in the potential range from 0.4 V to 0.9 V. The pSi-PPy membrane exhibited capacitive charging characteristic to the PPy, where the current quickly moved toward a constant value. They did not apply a higher voltage, preventing overoxidation or partial destruction of the polymer. The research team recorded the sample length change, for detailed characterization of electrochemical actuation while recording the CVs (cyclic voltammograms).
![Electrochemical actuation experiments. (A) Schematics of the electroactuation experiments on the pSi membrane (gray) filled with PPy (green) immersed in an aqueous electrolyte [HClO4 (blue and red) and H2O (red and white) molecules]. The dimensions of the as-fabricated membrane, on the left, are length l0, width w, and thickness t. The middle part illustrates the case where a voltage of 0.4 V is applied and the ClO−4 anions are expelled from the PPy, resulting in the contraction of the sample. Vice versa, in the right part, a voltage of 0.9 V is applied, and the anions are incorporated, followed by the subsequent expansion of the sample. The change in length is indicated by Δl. (B) The graph depicts an exemplary cyclic voltammetry of a pSi-PPy membrane in 1 M HClO4 electrolyte. The current j is plotted against the applied potential E measured versus the SHE. The potential sweep rate is 10 mV/s. (C) The graph depicts the mean values for the maximal current density of j plotted against varying potential sweep rates dE/dt from 10 to 50 mV/s. The dashed line indicates a linear regression of the data points, which yields the capacitance c* as the slope. Depicted on the right are (D) five representative potential cycles E, (E) the resulting volumetric charge qV, and (F) the introduced effective strain ε of the nanoporous membrane. Credit: Science Advances, doi: 10.1126/sciadv.aba1483 Giant electrochemical actuation in a nanoporous silicon-polypyrrole hybrid material](https://scx1.b-cdn.net/csz/news/800a/2020/2-giantelectro.jpg)
Step-coulometry
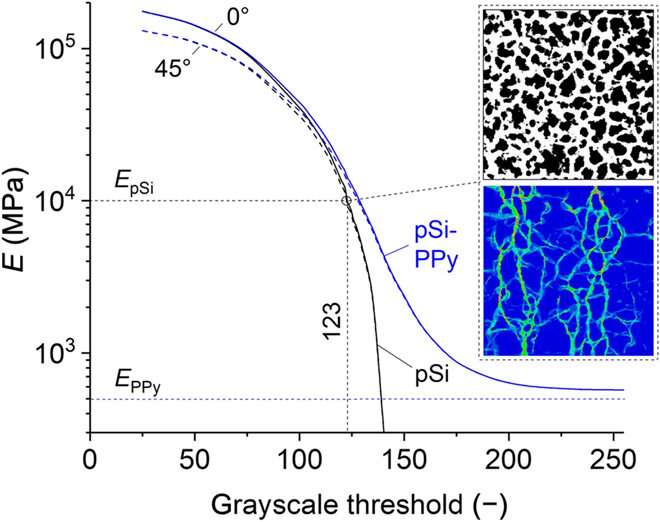
Brinker et al. then performed step coulometry to analyze the actuation kinetics and the amount of matter transformed during an electrolysis reaction by measuring the amount of electricity consumed or produced in the setup. The strain response of the experimental setup was faster than the charging and discharging process by almost an order of magnitude. Two effects may have contributed to the observation. First, during the experiment, the polypyrrole (PPy) may have reached its yield limit to cause plastic deformation. The whole sample will not expand further, despite the inclusion of counter ions into the polymer as noted through micromechanical analysis. Second, the diffusion limitations may have hindered the faster transfer of anions to the PPy, a kinetic limitation supported by molecular dynamics simulations. The scientists also modeled the micromechanical properties of the microstructure extracted from the electron micrograph of the same area of material to understand the mechanism of electroactuation of the PPy-filled pSi membrane. They measured the macroscopic Young's modulus of the material for the empty PPy and PPy-filled with pSi membrane to show how the structure of the pSi network dominated the macroscopic stiffness of the material.
Improved functionality of the biohybrid system
Internal mechanical swelling pressure in the aqueous electrolyte/PPy (polypyrrole) system contributed to the movement of counter ions into pore space due to the electrical potential applied to the entire porous medium. In contrast to piezoelectric materials, the potential applied in this work to obtain exceptional actuation using the biocompatible hybrid materials was significantly lower, evidencing improved functionality of the hybrid system. In this way, Manuel Brinker and colleagues integrated large electrochemical actuation into a mainstream semiconductor alongside functional integration of porous silicon (pSi) to establish versatile and sustainable pathways for electrochemical energy storage and other applications in aqueous electrolytic media. This work expanded on previous approaches on combining classic piezoelectric actuator materials, however, in contrast to high-performance piezoelectric ceramics, the team did not integrate any heavy metals such as lead (Pb) for functionality. The materials used in this work are biocompatible and biodegradable, alongside exceptionally small functional voltages suited for biomedical functions of actuation. From a materials science perspective, the research showed how self-organized porosity in solids could be functionalized to design robust, 3-D mechanical materials to integrate nanocomposites within macroscale devices.
More information: Manuel Brinker et al. Giant electrochemical actuation in a nanoporous silicon-polypyrrole hybrid material, Science Advances (2020). DOI: 10.1126/sciadv.aba1483
Wenfeng Liu et al. Large Piezoelectric Effect in Pb-Free Ceramics, Physical Review Letters (2009). DOI: 10.1103/PhysRevLett.103.257602
Amir H. Atabaki et al. Integrating photonics with silicon nanoelectronics for the next generation of systems on a chip, Nature (2018). DOI: 10.1038/s41586-018-0028-z
Journal information: Science Advances , Nature , Physical Review Letters
© 2020 Science X Network